Treatment of cancer with 2-deoxyglucose
A glucose and cancer technology, applied in the field of cancer treatment using 2-deoxyglucose, can solve unproven cancer and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
Growth inhibitory activity of 2-deoxy-D-glucose on human non-small cell lung cancer cell lines
[0101] In this example, the inhibitory effect of 2-deoxy-D-glucose (2-DG) on two cell lines obtained from human non-small cell lung cancer (NSCLC) was demonstrated. The human lung cancer cell lines in question are MV522 and NCI-H23, both obtained from tumors exhibiting adenocarcinoma histopathology, ie one of at least three types of NSCLC. The NCI-H23 tumor cell line is available from ATCC (Rockville, MD) and the MV522 cell line was provided by Dr. M.J. Kelner, University of California. at 37°C with 5% CO 2 Cells were cultured in RPMI medium (Nova Tech, Grand Island, NY) in a humid atmosphere. For passaging, at 75cm 2 Cells grown in shake flasks (60-70% confluent) were rinsed with PBS and removed from shake flasks using trypsin (Gibco BRL) prior to passaging. The cells used in the following experiments were centrifuged and washed at 10 5 Cells / ml were resuspended in cell cultur...
Embodiment 2
Evaluation of 2-DG as a Sole Agent and in Combination with Cisplatin and Paclitaxel
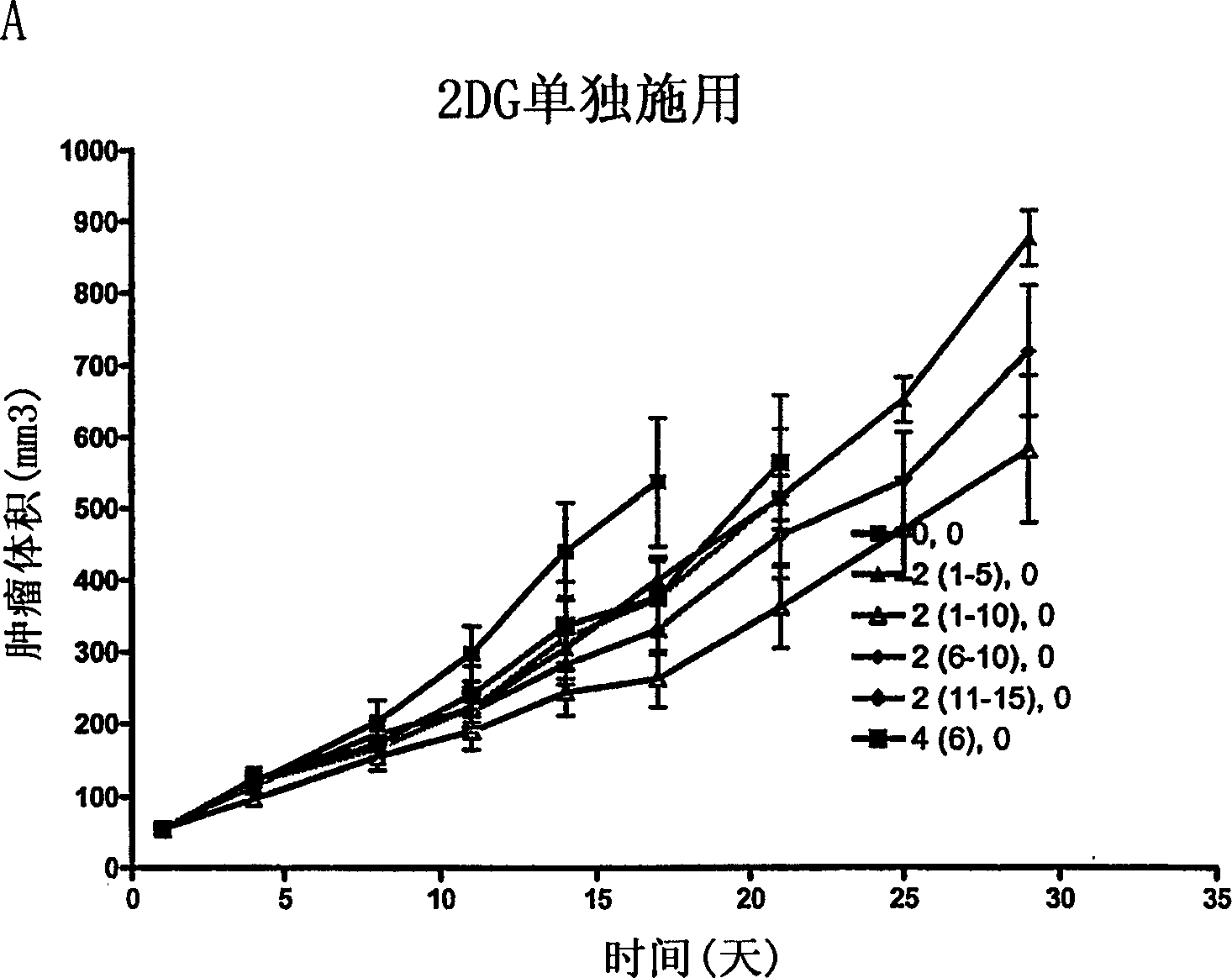
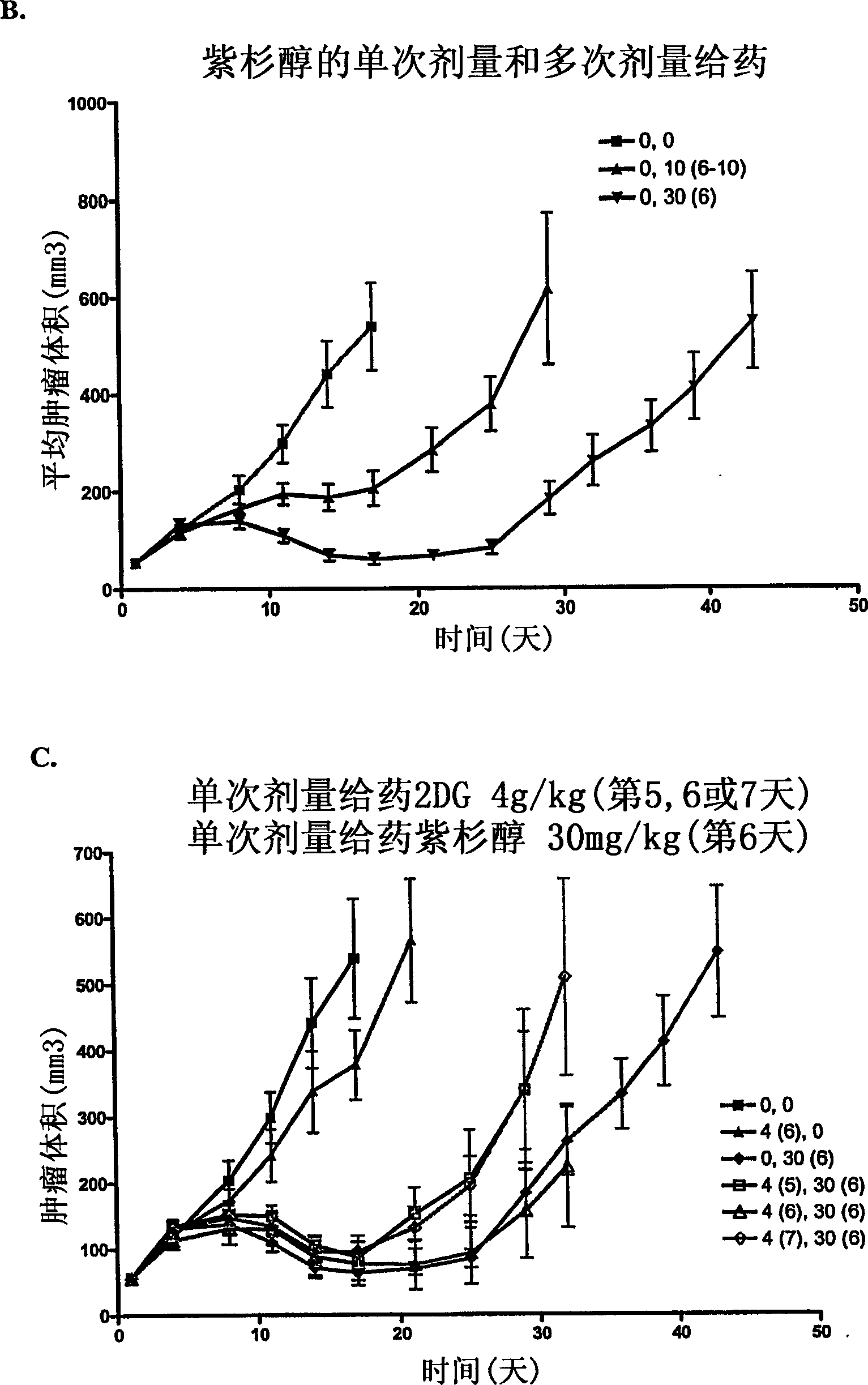
[0105]In the murine MV552 xenograft model, the effect of 2-deoxy-D-glucose (2-DG) alone and in combination with cisplatin was tested in a tumor growth delay study and compared with cisplatin alone The effects were compared as follows: 5-6 week old female nude mice weighing about 20 g were purchased from Harlan, Inc. (Madison, WI). Animals were implanted subcutaneously with sleeves of MV522 human tumor carcinoma fragments harvested from tumors grown subcutaneously in nude mouse hosts. When the tumor size was approximately 71 mg (11 days after inoculation), animals were pair-matched into treatment and control groups. Ten tumor-bearing mice were included in each group. Each mouse was ear-marked for follow-up throughout the experiment. The initial dose was given on day 1 after pair matching. The dosing schedule for 2-DG was oral (p.o.), twice daily, every 12 hours, × 5 until the end (twice dai...
Embodiment 3
Oral formulations of 2-DG
[0118] This example illustrates the preparation of a representative pharmaceutical formulation for oral administration.
[0119] A. Dispensing 2-DG into hard shell gelatin capsules containing 100 mg to 1 g of 2-DG; optionally, about 0.5% (w / w) magnesium stearate may be added. Additionally, a mixture of 2-DG and lactose is available in capsules.
[0120] B. Using 2-DG (20.0% to 89.9% wt / wt., depending on the presence and amount of lactose); magnesium stearate (0.9%); starch (8.6%); optionally lactose (0 -69.6%) and PVP (polyvinylpyrrolidone; 0.9%), other raw materials except magnesium stearate were mixed and granulated using water as a granulating liquid. The preparation is then dried, mixed with magnesium stearate and made into tablets with a tablet machine.
[0121] C. Dissolve 2-DG in a mixture of propylene glycol, polyethylene glycol 400, and polysorbate 80; add water; then dispense the resulting mixture into bottles.
[01...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com