Solanesyl bromine preparation process and its refining method
The technology of a preparation process and a purification method is applied to the preparation process of solanesyl bromide and the purification field thereof, and can solve the problems of reduced yield, easy decomposition, long column chromatography and the like, achieves large production capacity, and eliminates waste water discharge. Volume and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0017] Chemical reaction equation:
[0018]
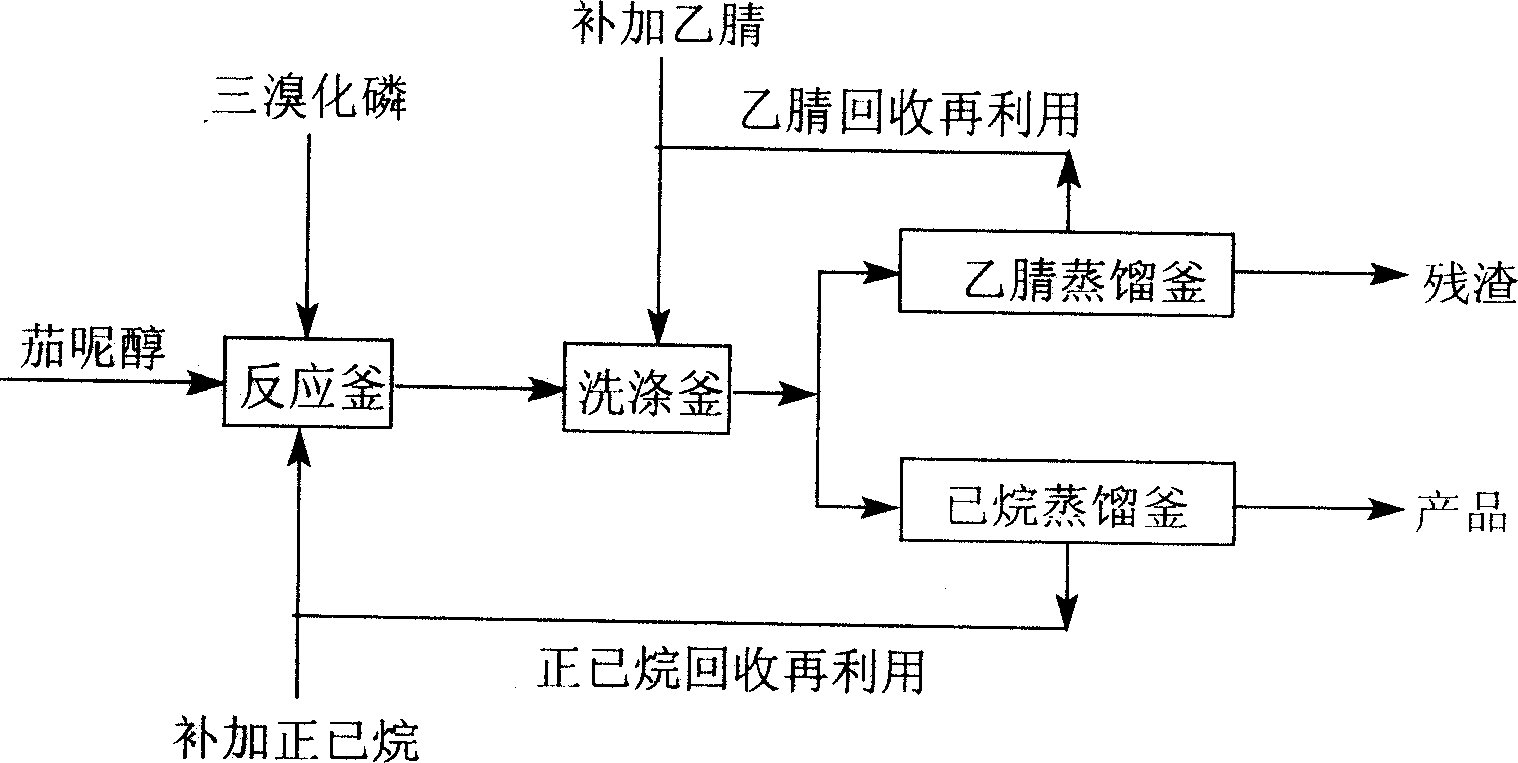
[0019] figure 1 In, solanesol 74g (purity 85%), 260ml n-hexane, 2.2ml pyridine, stirring and dissolving, temperature-10~10 ℃ under stirring, the 40ml n-hexane solution of 4.4ml phosphorus tribromide is added dropwise in 30 minutes, then The stirring reaction was continued at the same temperature for 2 hours. 200 ml of acetonitrile was added for washing three times, and the solvent was evaporated under reduced pressure to obtain 71 g of solanesyl bromide with a yield of 98% (calculated as pure solanesol) and a purity of 97%.
[0020] NMR (CDCl 3 ): δ1.63(24H, s), 1.68(3H, s), 1.73(3H, J=1Hz s), 1.96~2.10(32H, m), 4.02(2H, d, J=8.3Hz), 5.10 ~5.13 (8H, m), 5.53 (1H, t, J=8.3Hz).
[0021] Stir and dissolve 53g solanesol in a mixed solvent of 50ml petroleum ether (b.p40~45℃) and 70ml anhydrous ether, then add 1.8ml pyridine, stir at 0~10℃ for 1 hour, add dropwise 3.5ml phosphorus tribromide 20ml of petroleum ether solution, then...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com