Intercalator of slow-releasing food preservative substance, and its prepn. method
A technology for food preservatives and intercalation materials, which is applied in the fields of food preservation, food science, and applications, and can solve problems such as no reports of preservative molecular intercalation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] A. Use deionized water and Zn(NO 3 ) 2 ·6H 2 O, Al(NO 3 ) 3 9H 2 O and sorbic acid SA prepare 300ml mixed solution, make Zn(NO 3 ) 2 ·6H 2 O, Al(NO 3 ) 3 9H 2 The molar concentrations of O and SA were 0.4, 0.2 and 0.8 mol / L respectively; another 300ml NaOH alkali solution was prepared and the molar concentration of NaOH was 2 mol / L.
[0038] B.N. 2 Under protection, the alkali solution is gradually added dropwise into the vigorously stirred mixed solution, and the pH value of the slurry is controlled to be 7-9 after the dropwise addition is completed.
[0039] C. Put the resulting slurry in N 2 Crystallize at 60°C for 18 hours under protection, then filter with suction, wash with water until neutral, and dry at room temperature for 2 days to obtain SA-ZnAl-LDHs. The water used in the process is decarbonized deionized water.
[0040] The crystal structure of the product is analyzed by XRD, showing that it has a typical organic intercalation hydrotalcite mat...
Embodiment 2
[0047] With deionized water, Zn(NO 3 ) 2 ·6H 2 O, Fe(NO 3 ) 3 9H 2 O, Al(NO 3 ) 3 9H 2 O and lactic acid (LA) prepare 300ml mixed solution, make Zn(NO 3 ) 2 ·6H 2 O, Fe(NO 3 ) 3 9H 2 O, Al(NO 3 ) 3 9H 2 The molar concentrations of O and LA were 0.4, 0.1, 0.1 and 1.0 mol / L, respectively. Other steps are the same as in Example 1 to obtain LA-ZnFeAl-LDHs.
[0048] The crystal structure of the product is analyzed by XRD, showing that it has a typical organic intercalation hydrotalcite material crystal structure ( figure 2 )
[0049] The product is analyzed by TG / DTA, ICP and elemental analysis methods, and the main composition of the obtained product is as follows:
[0050] Zn 31.8%
[0051] Fe 8.1%
[0052] Al 3.8%
[0053] LA 26.2%
[0054] This example demonstrates that preservative LA intercalated LDHs can be obtained by co-precipitation method.
Embodiment 3
[0056] The preservative SA in Example 1 was replaced with LA, and the other steps remained unchanged to prepare LA-ZnAl-LDHs.
[0057] The product is analyzed by TG / DTA, ICP and elemental analysis methods. The main components of the product are as follows:
[0058] Zn 33.1%
[0059] Al 7.6%
[0060] LA 28.6%
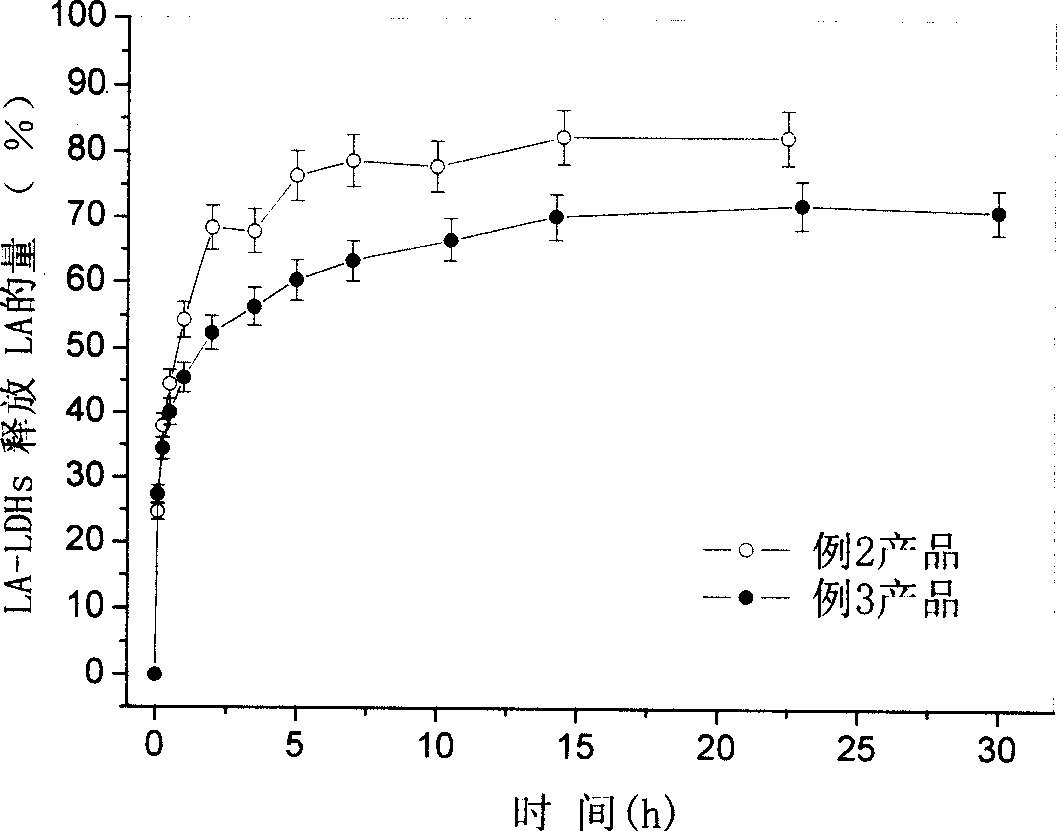
[0061] Get each 0.5g of the product of embodiment 2 and embodiment 3, be dispersed in the 100ml sodium carbonate solution in the Erlenmeyer flask respectively. Then put the Erlenmeyer flask into a water bath constant temperature shaker and shake continuously. A small amount of suspension was taken at a certain time interval, and after the suspension was centrifuged, the concentration of LA in the supernatant was determined by reverse-phase high-performance liquid chromatography. In this way, the slow release performance of the product was investigated. The rate figure that the product of embodiment 2 and embodiment 3 releases LA in sodium carbonate solution sees i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com