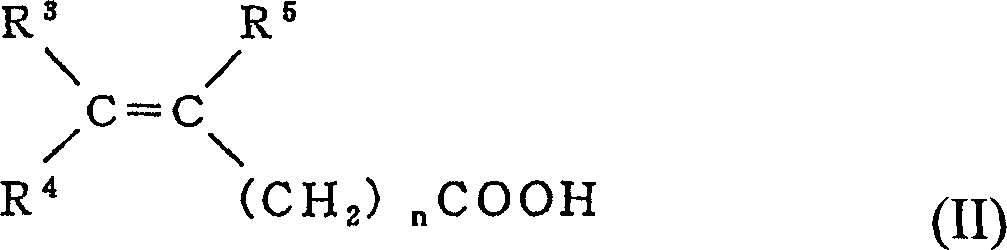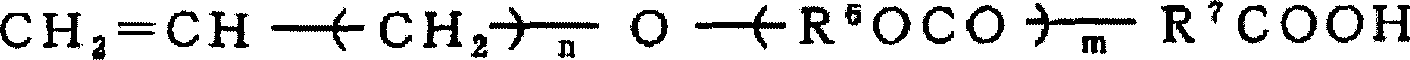Fluorine-containing aqueous coating composition
A technology of water-based coatings and compositions, applied in the direction of polyurea/polyurethane coatings, coatings, etc., can solve the problems of low compatibility, water resistance of coating films, solvent resistance and pollution resistance, insufficient hardness, low crosslinking density, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0079] The preparation of the fluorine-containing polymer (a1) is the same as the above-mentioned fluorine-containing olefin copolymer (A1) containing a functional group, preferably by a solution polymerization method. If prepared by emulsion polymerization, the polymerization is insufficient due to the low polymerizability of fluorine-containing olefins, the low polymerizability of monomers containing carboxyl and hydroxyl groups, the large composition distribution of the polymer, and the need for a large amount of emulsifier.
[0080] The solution polymerization method, polymerization conditions, organic solvent, polymerization initiator, molecular weight regulator, and the like are preferably those exemplified in the above-mentioned functional group-containing fluorine-containing olefin copolymer (A1).
[0081] A compound (a2) having a functional group that can react with a carboxyl group or a hydroxyl group and a polymerizable unsaturated double bond is reacted with the obt...
Synthetic example 1
[0154] 1.6 kg of acetone, 500 g of isopropanol, and 680 g (6.8 mol) of tetrafluoroethylene were added to a 6-liter stainless steel autoclave, and after nitrogen exchange, the temperature was raised to 67.5°C. Add 60g octanoyl peroxide solution (solid content 30%) under stirring state, then add 178g (2.5 moles) ethyl vinyl ether, 287g (2.5 moles) hydroxybutyl vinyl ether, 250 g (1.2 mol) of a mixture of 3-(2-allyloxyethoxyl)propionic acid [=reaction product of ethylene glycol monoallyl ether and succinic anhydride]. After the monomer addition was complete, an additional 60 g of the same peroxide solution was added two hours later. After further continuing to stir for 2 hours, the temperature was raised to 80° C., and stirred for 3 hours. The reaction mixture was cooled to room temperature, and the unreacted monomers were removed, followed by nitrogen exchange to obtain 3.4 kg of the product (36.9% by weight of solid content). The composition of the obtained copolymer was 50 m...
Embodiment 1
[0156] Put 50g of the above-mentioned F-1 into a 100ml plastic cup (polycap), then add 0.5g thickener (プライマル QR708), 0.25g defoamer (Byk023), 0.15g sulfate ester type anionic surfactant (Japan Emulsifier Co., Ltd. (Newcol-707SF) and 5.0 g of ion-exchanged water were stirred at 1000 rpm for 20 minutes with a homogenizer, and left overnight. Add 14.5g of ethylene oxide-modified non-blocking isocyanate compound (Bayhiju-Lu 3100), 3.5g of methoxypropyl acetate, slowly add 20g of ion-exchanged water while stirring at 2000rpm, about Stir for 5 minutes. This mixture was filtered through a sieve, and coated with an applicator on an aluminum panel previously coated with a solvent-soluble cross-linked fluorine-containing paint (white) and sanded to prepare a coated panel. This coated panel was allowed to stand at room temperature for 5 days to be cured, and a test piece was prepared to check the following properties. The results are shown in Table 1.
[0157] Appearance: Visually obs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydroxyl value | aaaaa | aaaaa |
| Hydroxyl value | aaaaa | aaaaa |
| Acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com