Phentriazine derivative preparation and uses
A technology of benzotriazine and derivatives, which is applied in the field of compound preparation methods and can solve problems such as toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
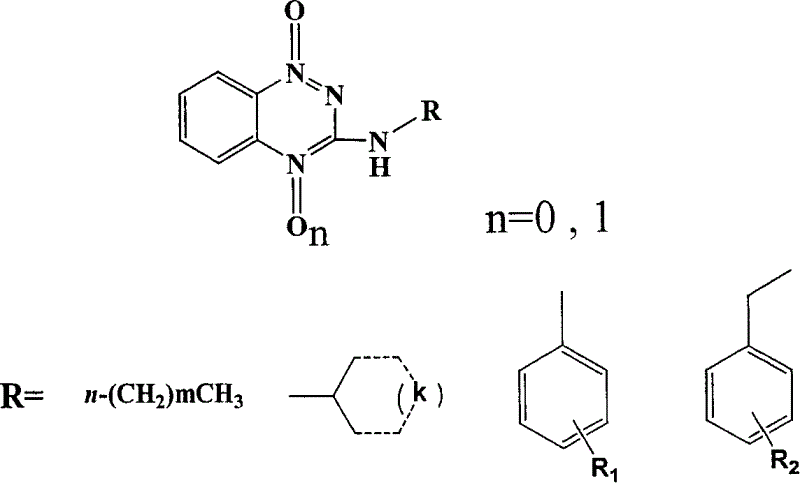
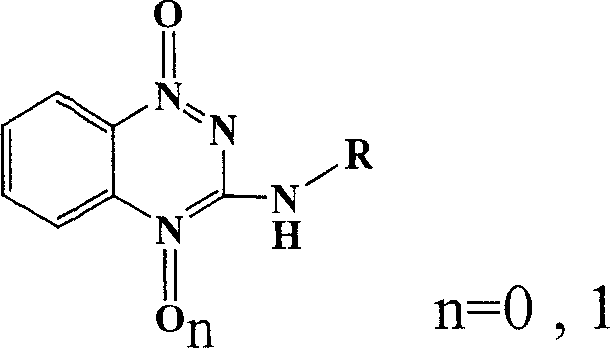
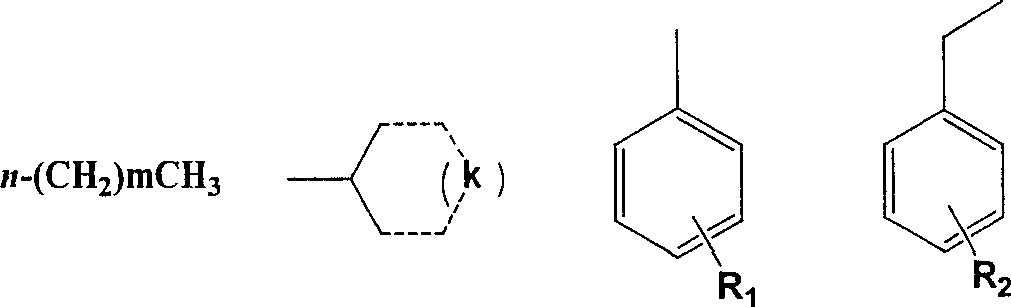
[0025] Detailed steps of the target compound: 39.6g (0.13mol) of triphosgene was dissolved in 30ml of toluene, and 27.6g (0.2mol) of o-nitroaniline (I) and 27.6ml (0.2mol) of triethylamine were added dropwise under the protection of nitrogen at room temperature. The toluene solution in 30min was added dropwise, and the temperature was raised to the reflux temperature of toluene. After 3h, o-nitroisocyanate (II) was obtained. After cooling, anhydrous ammonia gas was passed directly into it for 30min without separation, filtered, and washed with petroleum ether and hot water in turn. 30.4 g of o-nitrourea (III) was obtained, and 3-hydroxyl-1,2,4-benzotriazine-1-oxide (IV) 17.3 g was obtained by ring closure with 30% sodium hydroxide, 3-hydroxyl- 1,2,4-Benzotriazine-1-oxide (IV) with POCl 3 Substitution gives 3-Cl-1,2,4-benzotriazine-1-oxide (V) 16.6g, V and C 4 -C 10 fatty amine, C 3 -C 6 Condensation of cycloalkylamines, substituted arylamines and substituted benzylamines t...
Embodiment 2
[0027] 3-Hydroxy-1,2,4-benzotriazine-1-oxide (IV): prepared by referring to the literature method (F. J. Wolf et al, J. Amer. Chem. Soc., 1954, 4611-4613).
[0028] 1 H NMR (DMSO, AM=400): δ8.07(d, 1H), 7.76~7.80(t, 1H), 7.28~7.34(q, 2H), 3.34(s, 1H)
Embodiment 3
[0030] 3-Cl-1,2,4-Benzotriazine-1-oxide (V): Prepared by reference method (R.F. Robbins et al, J. Chem. Soc., 1957, 3186-3194).
[0031] 1 H NMR (CDCl 3 , AM=400): δ8.44~8.46(d, 1H), 8.02~8.03(d, 2H), 7.78~7.81(q, 1H)
[0032] MS(EI): MW=181, 183(34.85), 181(100), 137(10.53), 116(13.60), 102(6.91), 90(83.15), 76(29.81), 63(51.70), 50 (26.52), 43 (2.70)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com