Synthesis method of ranitidine alkali and its hydrochloride
A technology of ranitidine base and synthesis method, which is applied in the synthesis field of ranitidine base and its hydrochloride, can solve the problems of residue, influence, increased cost and the like, and achieves low cost, simple process and easy industrial production. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
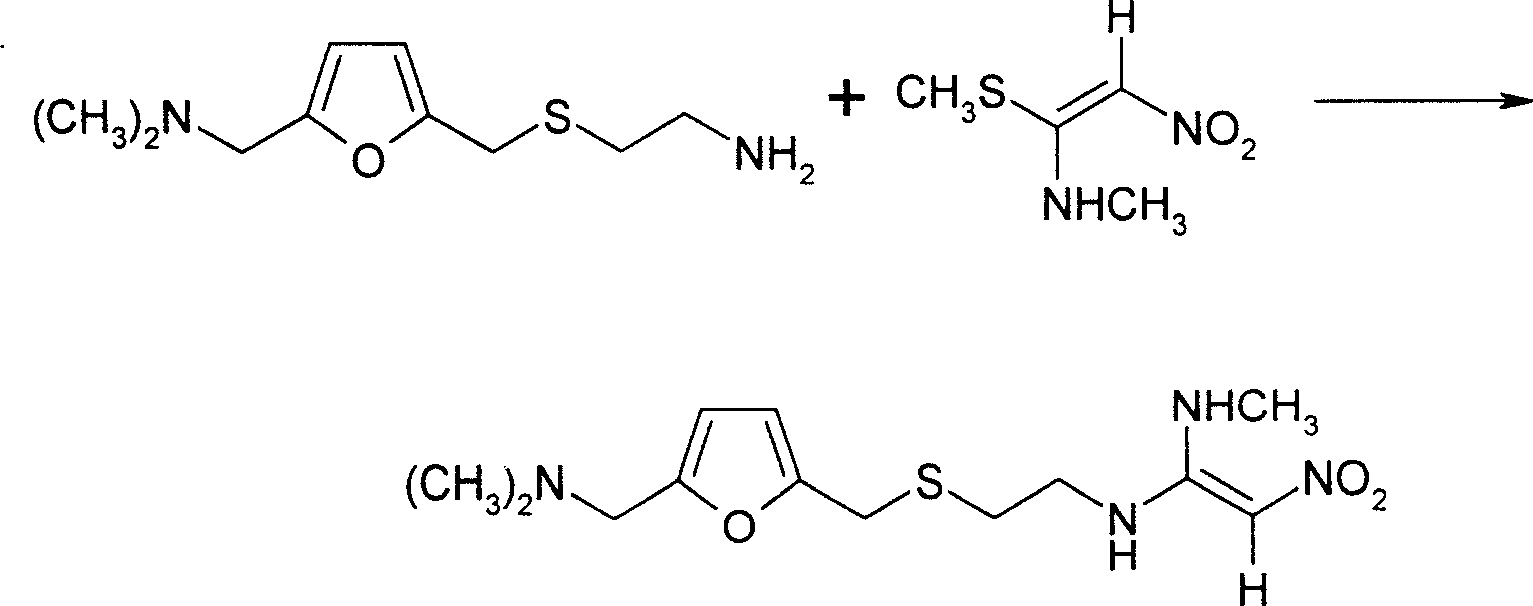
[0016] 1. Add 367.5kg of 2-[[[5-(dimethylamino)methyl-2-furyl]methyl]thio]ethylamine and 655kg of purified water into the reaction tank, and add 1-methylthio under stirring - 245kg of 1-methylamino-2-nitroethylene, the molar ratio of the two reactants is 1.04:1, the temperature is slowly raised to 48-52°C, the vacuum degree is controlled at 0.02-0.05Mpa, and the reaction is kept for 4.5 hours, and the reaction is completed.
[0017] Use cold brine to cool the feed liquid to about 25°C-35°C, adjust the base with 10% sodium hydroxide solution, and measure it with a pH meter until pH = 11.00-11.40. Crystallize at ~2°C, freeze for 12 hours and filter, rinse the filter cake with 50-70kg of purified water per centrifuge to obtain the wet product of ranitidine base. The reaction equation is as follows
[0018]
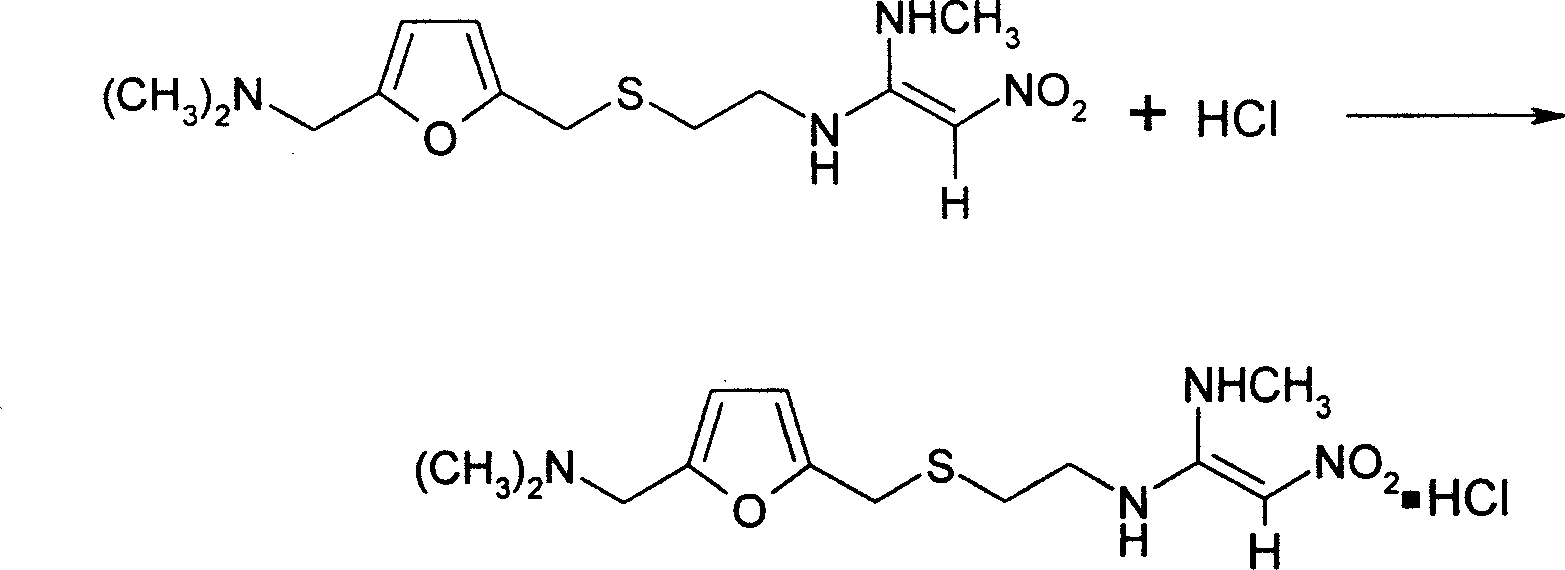
[0019] 2. Put the wet ranitidine base into the concentration tank, the temperature in the tank is below 65°C, and the vacuum degree is 0.09-0.098Mpa, distill water, after...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com