Preparation method of carbonyl nickel, nickel powder and its usage
A technology of nickel source and product, applied in the field of producing nickel carbonyl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1 (prior art)
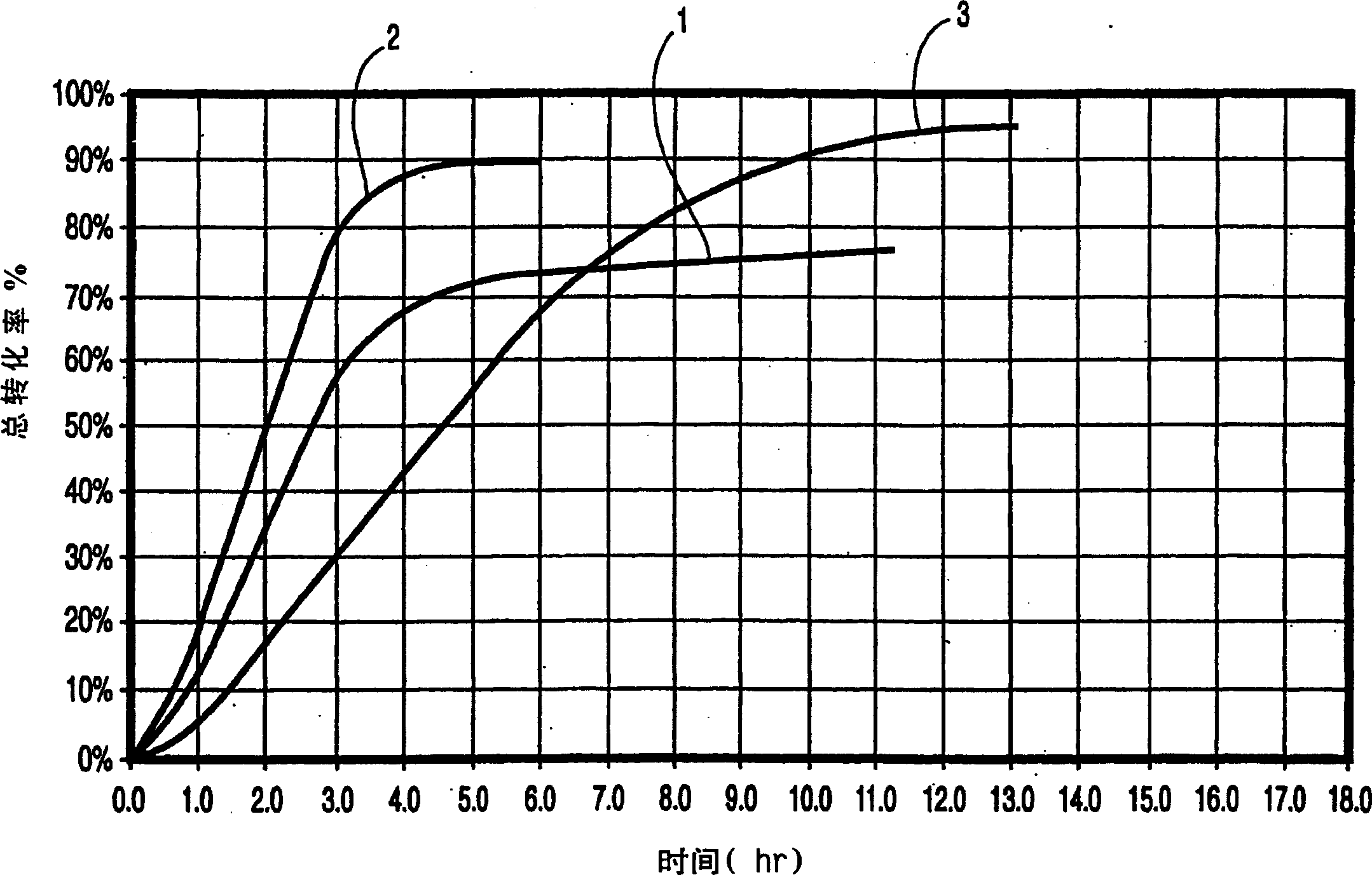
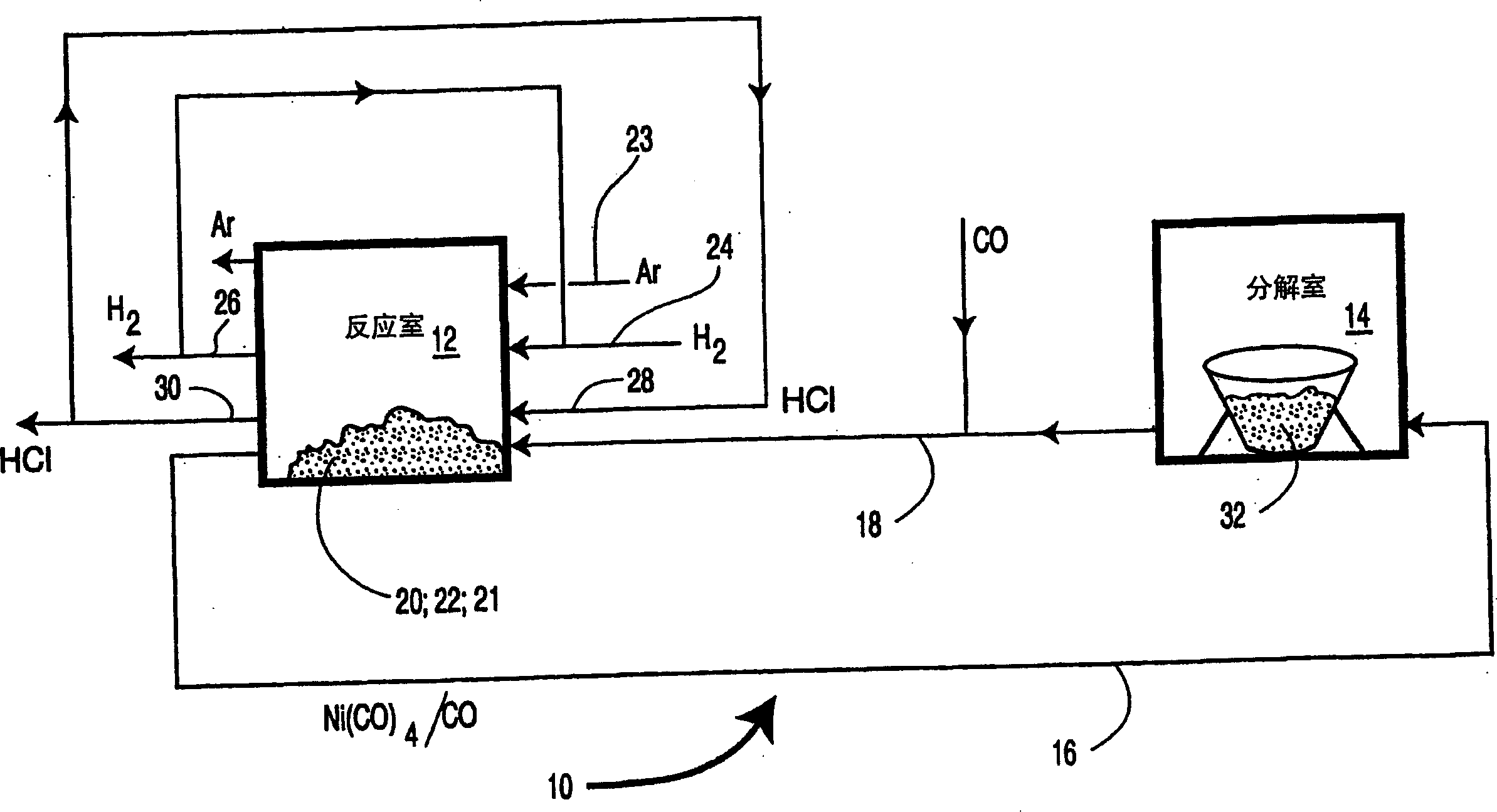
[0047] NiCO 3 powder (200g) into the extraction reactor, and then use H 2 The gas stream was treated at 500°C for 7 hours at a flow rate of 300 mL / min for essentially complete reduction. Cool the produced nickel powder to 100°C, then replace H with carbon monoxide 2 atmosphere. The reactor was further cooled to 50° C. and bubbled with CO gas at a flow rate of 300 mL / min. The resulting Ni(CO) 4 Nickel (10 g, 10% yield) was recovered as nickel powder after 12 hours by passing through a pair of carbonyl decomposers according to the prior art.
Embodiment 2
[0048] Embodiment 2 (prior art)
[0049] Ni(OH) 2 powder (100g) into the extraction reactor, and then 2 The gas stream was treated at 500°C for 7 hours at a flow rate of 300 mL / min for essentially complete reduction. The resulting nickel powder was placed in H 2 Cool to 100°C in atmosphere, then replace H with carbon monoxide 2 atmosphere. The reactor was further cooled to 50° C. and bubbled with CO gas at a flow rate of 300 mL / min. The resulting Ni(CO) 4 The gas was passed to the carbonyl decomposer and nickel (6 g, 9.5% yield) was recovered as nickel powder after 12 hours.
Embodiment 3
[0051] 300.1 g of nickel carbonate / nickel chloride mixture (10:1 w / w) was put into the extraction reactor, and treated with hydrogen (2 L / min) at 450° C. for 6 hours. Subsequently, the hydrogen was replaced with argon, the reactor was cooled to 40 °C and the argon was replaced with carbon monoxide at a gas temperature of 80 °C and a flow rate of 4 L / min, resulting in the formation of nickel carbonyl, which was collected and subsequently decomposed into Ni and CO at 6 103 g of nickel powder was provided within 1 hour, corresponding to a yield of 70% of the nickel extraction yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com