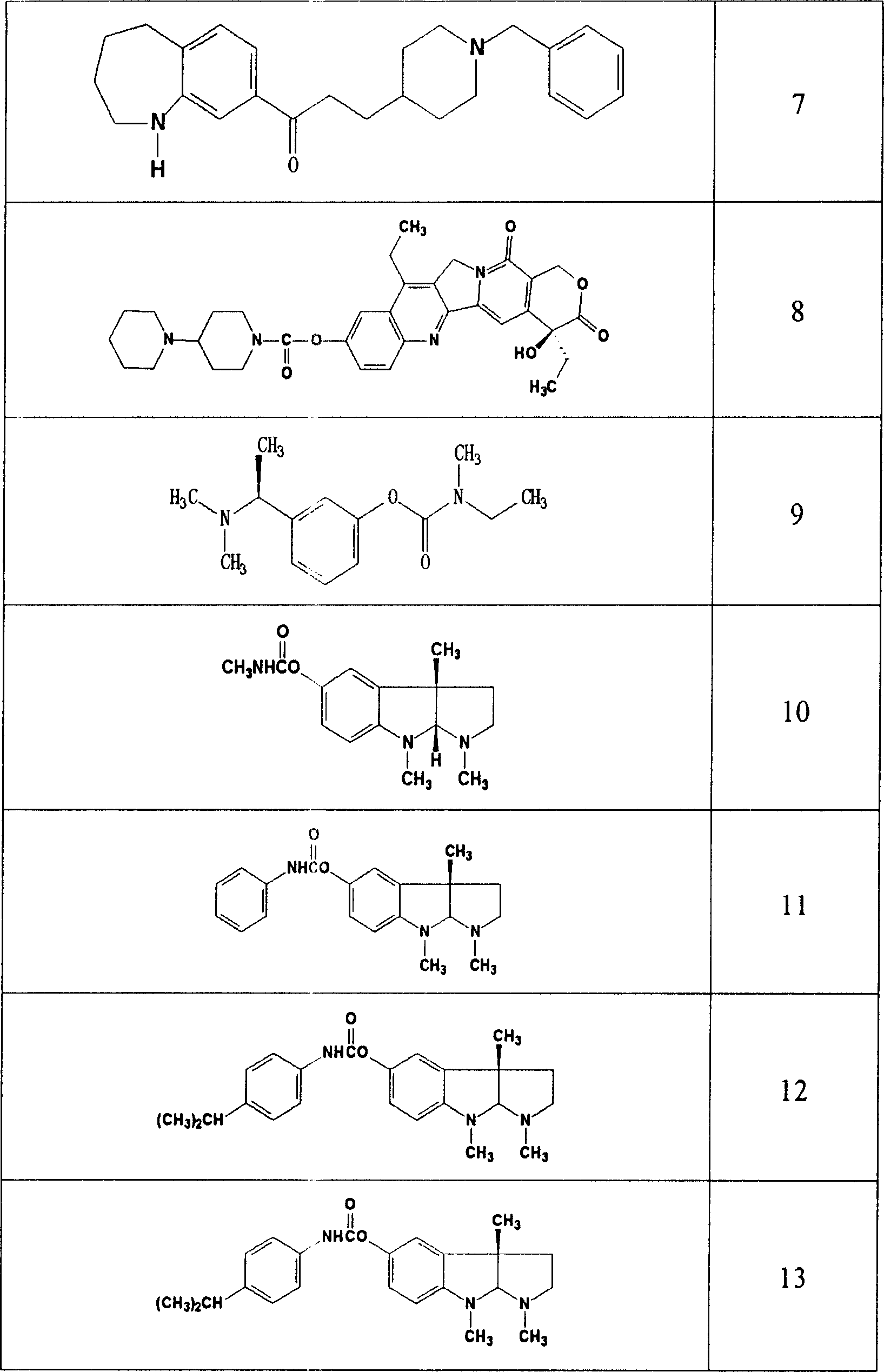
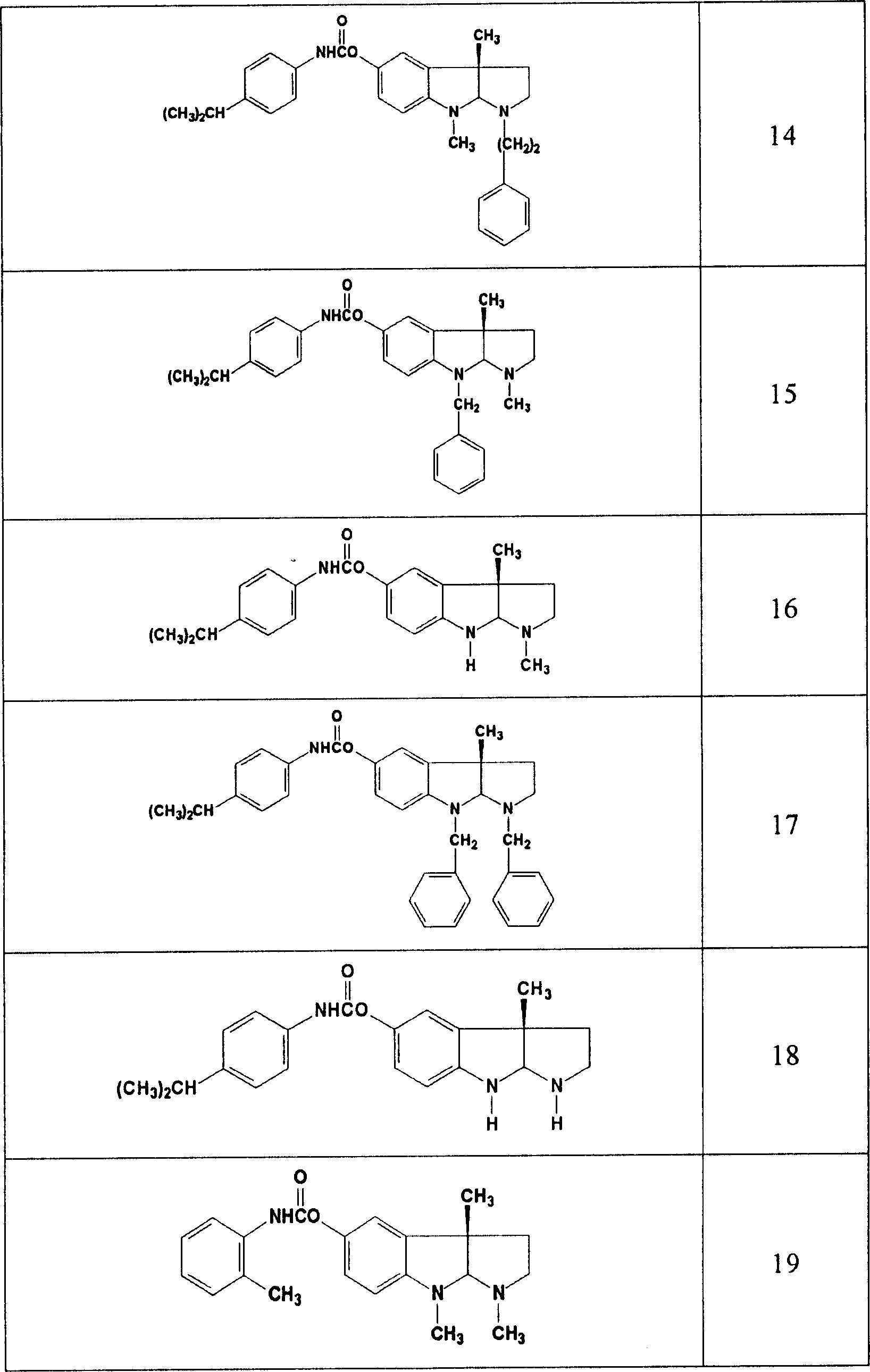
Use of acetylcholinesterase inhibitor for preparing diabetes medicines
An acetylcholinesterase and diabetes drug technology, applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, etc., can solve the problems of lactic acidosis, loss of reaction, hypoglycemia, etc., and achieve the effect of improving the symptoms of hyperglycemia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0160] The preparation of embodiment 1 diabetes model
[0161] C57BL / 6J mice aged 6-8 weeks were reared routinely. Before injection, STZ was dissolved in 0.1M sodium citrate buffer (PH4.5), and 45 mg / kg was injected intraperitoneally continuously for 5 days. Check your blood sugar twice a week with a Roche blood glucose tester. When the blood glucose concentration is greater than 13.9mM (250mg / ml), it is considered that the diabetes model has been established.
Embodiment 2
[0162] EXAMPLE 2 Expression of AChE in the apoptotic β-cell in the diabetes model in vitro
[0163] 1. STZ can induce RIN-5F cell apoptosis in vitro
[0164] Rat insulinoma cell line RIN-5F was incubated with 1640 medium (15% calf serum, 1.0mM sodium pyruvate, 4.5g / l glucose, 10mM HEPES) at 37°C, 5% CO 2 to cultivate. When the cell growth reached 70% density, after induction with 5mMSTZ, 10mM STZ, 15mM STZ for 24h, the apoptosis was detected by MTT method, flow cytometry and DNA ladder.
[0165] Results: MTT method was used to find that different doses of STZ could reduce the activity of insulinoma cell line RIN-5F ( figure 2 A). 16mM STZ can reduce cell viability to 40%. Flow cytometry also found that with the increase of STZ amount, the rate of apoptosis also increased. Normal cells have about 5% apoptosis rate, and after 24h treatment with 15mM STZ, the apoptosis rate can increase to 52% ( figure 2 B). After inducing RIN-5F cells with 5mM STZ for 24h, it can be fou...
Embodiment 3
[0181] Example 3 Expression of AChE in β-cells of diabetic model mice
[0182] After sacrificing the experimental mice, the pancreas was fixed with 4% paraformaldehyde (PFA) solution at 4°C for 24 hours, then soaked in 30% sucrose solution for 12 hours, embedded with OCT, frozen sections at 10 μM, and stained with acetylcholinesterase.
[0183] Results: In STZ-induced diabetic model islet slices, β-cells had obvious AChE activity. There was no positive staining of AChE in pancreas sections of normal mice ( Figure 5 A). In contrast, with the apoptosis of β-cells and the infiltration of lymphocytes (from day 8 to day 29) in the course of diabetes, specific staining of AChE could be found around the islets ( Figure 5 B-E). Especially when the symptoms of diabetes are severe (from the 22nd day to the 29th day), the destruction of β cells is already quite serious, and the staining of AChE is very strong ( Figure 5 D, E).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com