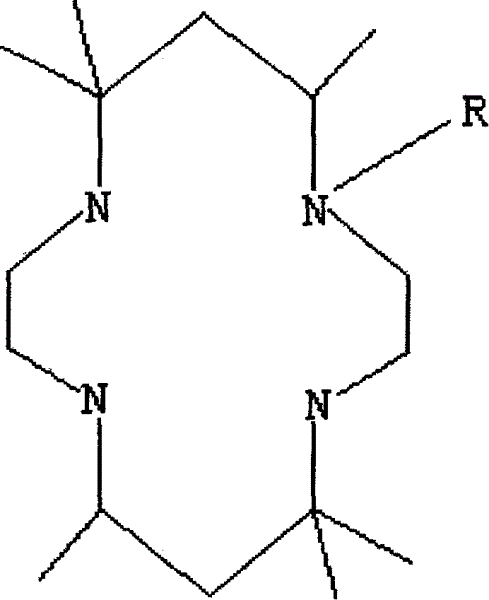
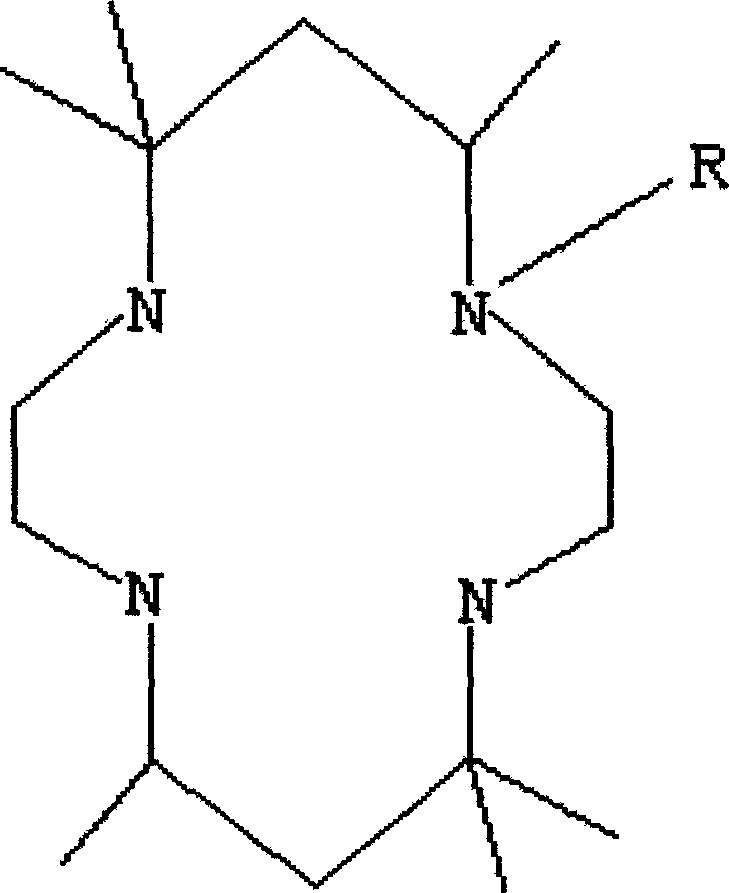
Tetraazacyclo tetradecane derived ligand complex and its synthesis process
A technology of tetraaza heterocycle and synthesis method, which is applied in the field of macrocyclic ligand complexes and synthesis, can solve the problems of industrial application obstacles and high preparation cost, and achieves the effects of easy industrial production, low production cost and environmental improvement.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The implementation process of the present invention will be further elaborated below through specific examples.
[0022] 1. Synthesis of 5,5,7,12,14,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane
[0023] Synthesized according to the method of J.Chem..Soc.Perkin I, 591-593,1975.
[0024] 2. Synthesis of 1-acetyl-5,5,7,12,14,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane
[0025] Dissolve 28.5 grams (0.1 mol) of 5,5,7,12,14,14-hexamethyl-1,4,8,11-tetraazacyclotetradecane in 500 milliliters of dichloromethane, add 20 grams (0.14 mol) anhydrous potassium carbonate, stirred and cooled to 0°C. A solution of 7.9 g of acetyl chloride dissolved in 100 ml of dichloromethane was added dropwise in one hour. After the addition, the temperature was gradually raised to room temperature, and stirring was continued for 3 hours, then heated to reflux for 3 hours, and the reaction was completed and cooled to room temperature. After filtration, the filtrate was concentrated by distil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com