4-oxoquinoline compounds and utilization thereof as HIV integrase inhibitors
A kind of technology of oxoquinoline, compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific example
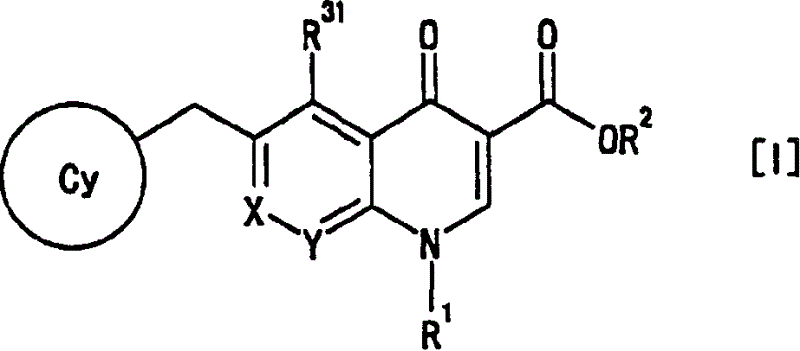
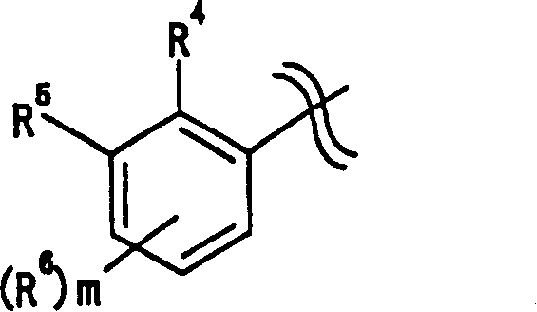
[0372] Specific examples thereof include 2-fluoroethyl, 2-chloroethyl, 2-bromomethyl, 3-fluoropropyl, 3-chloropropyl, 4-fluorobutyl, 4-chlorobutyl, trifluoromethyl , 2,2,2-trifluoroethyl, 3,3,3-trifluoropropyl, 4,4,4-trifluorobutyl, pentafluoroethyl, 2,2,2-trifluoro-1- Trifluoromethyl-ethyl etc.
[0373] as R 31 , R 4 , R 5 , R 6 , R 6′ , R 6″ , R 6 And group A, preferably trifluoromethyl.
[0374] "C 1-4 Alkoxy" is "C" in which the alkyl moiety is defined above 1-4 The alkoxy group of "alkyl", its specific examples are methoxy, ethoxy, propoxy, isopropoxy, butoxy, isobutoxy, tert-butoxy and the like.
[0375] for R 31 , preferably methoxy.
[0376] "C 1-4 Alkylthio" is "C" in which the alkyl moiety is defined above 1-4 An alkylthio group of "alkyl". Specific examples thereof include methylthio, ethylthio, propylthio, isopropylthio, butylthio, isobutylthio, tert-butylthio and the like.
[0377] for R 31 , preferably methylthio.
[0378] "Halo C 1-4 Alkoxy" i...
reference example 1
[0594] Preparation of 2,3-Dichlorobenzylzinc Chloride in THF
[0595]
[0596] To a suspension of zinc powder (55.1 g, 843 mmol) in tetrahydrofuran (THF; 56 ml) was added 1,2-dibromoethane (2.9 ml, 33.8 mmol) under argon flow and the mixture was heated to reflux for 5 minutes. Then, trimethylsilyl chloride (8.6ml, 67.5mmol) was added at 0°C, and the mixture was stirred at 0°C for 5 minutes, after which 2,3-dichlorobenzyl chloride was added dropwise under ice-cooling (82.4g, 421.7mmol) in THF (330ml). After the dropwise addition was completed, the mixture was warmed to room temperature and stirred for 1 hour to obtain a THF solution of 2,3-dichlorobenzylzinc chloride.
Embodiment 1-1
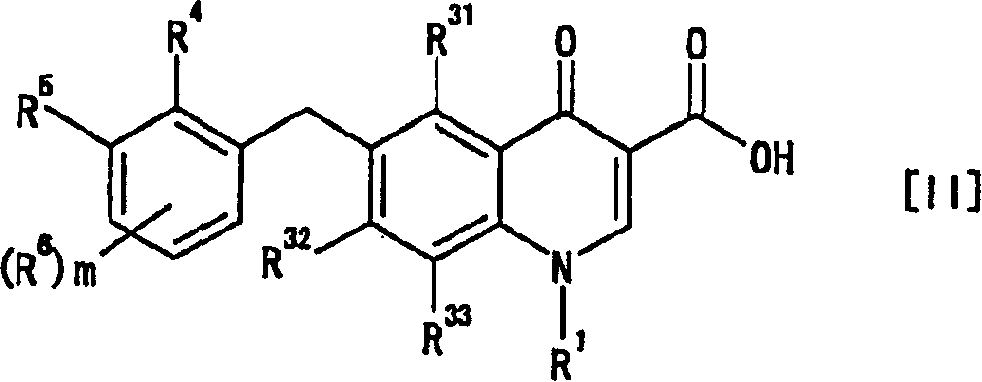
[0597] Example 1-1 Synthesis of 6-(2,3-dichlorobenzyl)-1,4-dihydro-1-(2-hydroxyethyl)-4-oxo-3-quinolinecarboxylic acid
[0598] step 1 Synthesis of 1,2-dichloro-3-(4-nitrobenzyl)benzene
[0599]
[0600] Bis(dibenzylideneacetone)palladium(0) (3.2 g, 5.6 mmol) and tris(2-furyl)phosphine (2.6 g, 11.2 mmol) were dissolved in THF (310 ml) under argon flow. To this solution, a THF solution of 2,3-dichlorobenzylzinc chloride (421.7 mmol) obtained in Reference Example 1 was added dropwise under ice cooling by cannula, followed by 4-iodonitrobenzene (70.0 g, 281 mmol) in THF (700 ml). After stirring at room temperature for 2 hours, to the reaction solution was added a saturated aqueous ammonium chloride solution, and the mixture was filtered through celite. The filtrate was concentrated under reduced pressure. Water was added to the residue and extracted with ethyl acetate. The organic layer was washed with water and saturated brine, and dried over sodium sulfate. After fil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com