Mixed compositions for controlling parasitic insects
A composition and technology for ectoparasites, applied in the directions of drug combinations, active ingredients of heterocyclic compounds, applications, etc., can solve problems such as difficulty in preventing and controlling drug-resistant ectoparasites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] The preparation method of formula (I) compound
[0054] Compounds of formula (I) can be prepared, for example, according to the methods shown in Scheme I. For the preparation of these compounds, Japanese Patent Unexamined Publication No. 128355 / 1991 can be referred to.
[0055] plan 1
[0056]
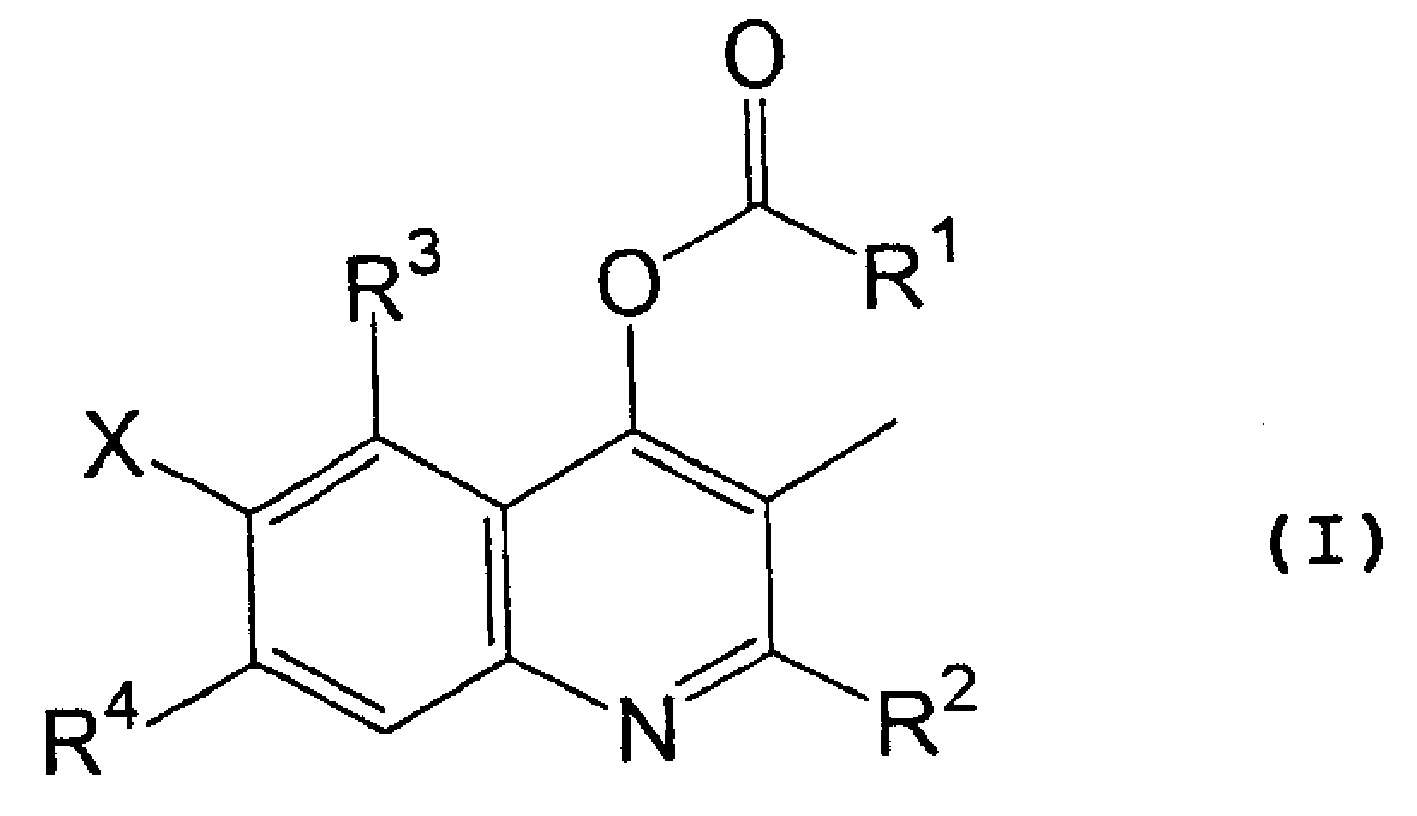
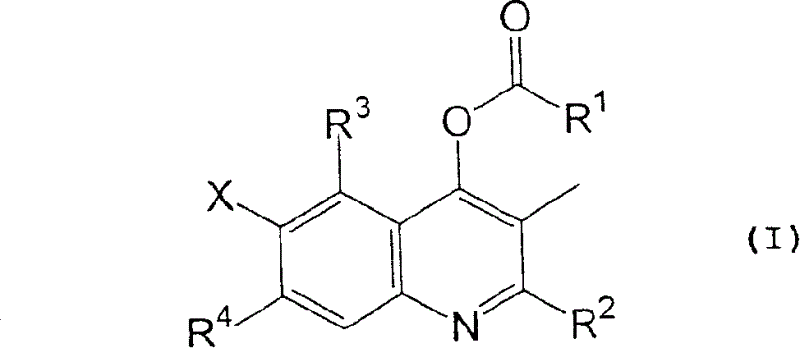
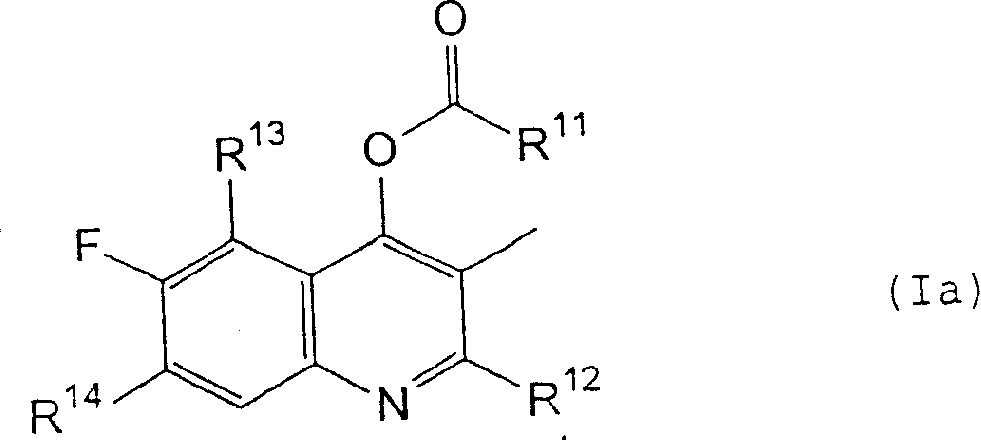
[0057] wherein Y represents a chlorine atom or a hydroxyl group; and R 1 , R 2 , R 3 , R 4 and X are as defined in formula (I).
[0058]A compound of formula (I) can be synthesized by reacting a compound of formula (II) with a reagent represented by formula (III) in the absence of a solvent or in the presence of a suitable solvent. When Y represents a chlorine atom, the compound of formula (I) can be synthesized by reacting in the presence of a suitable base, such as an organic amine such as triethylamine or pyridine, or an inorganic base such as sodium carbonate, potassium carbonate or sodium hydride . When Y represents a hydroxyl group, the compound of formula (...
Embodiment 1
[0115] Example 1: 2-ethyl-3-methyl-4-cyclopropanecarbonyloxy-6,7-difluoroquinoline (compound 1) and 2- Ethyl-3-methyl-4-cyclopropanecarbonyloxy-5,6-difluoroquinoline (compound 2)
[0116] 3,4-Difluoroaniline (3.18 g) and 3.9 g ethyl 2-methyl-2-propanoyl acetate were refluxed in toluene (50 ml) in the presence of 0.3 ml boron trifluoride etherate for 3 hours. The resulting reaction mixture was washed with saturated sodium bicarbonate solution and saturated brine and dried over anhydrous sodium sulfate, and the solvent was evaporated off. The resulting intermediate was refluxed in diphenyl ether for 30 minutes and left to cool. The precipitate was then collected by filtration under reduced pressure to obtain 1.9 g of 2-ethyl-3-methyl-4-hydroxyl-6,7-difluoroquinoline and 2-ethyl-3-methyl-4-hydroxyl-5 , a mixture of 6-difluoroquinolines. 60% Sodium hydride (20 mg) was suspended in 2 ml of dimethylformamide. Alternatively, a mixture of 2-ethyl-3-methyl-4-hydroxy-6,7-difluoro...
Embodiment 2
[0120] Example 2: 2-ethyl-3-methyl-4-acetoxy-6,7-difluoroquinoline (compound 3)
[0121]2-Ethyl-3-methyl-4-cyclopropanecarbonyloxy-6,7-difluoroquinoline (5.5 g), prepared as described in Example 1, was dissolved in 50 ml of methanol. A solution of 2.5 g of sodium hydroxide in 50 ml of water was added to the solution, and the mixture was stirred at 50°C for 3 hours. The resulting reaction solution was allowed to stand to cool, and then poured into 50 ml of water. The mixture was neutralized with 1N hydrochloric acid, and the precipitate was collected by filtration to obtain 5.1 g of 2-ethyl-3-methyl-4-hydroxy-6,7-difluoroquinoline. 60% sodium hydride (96 mg) was suspended in 20 ml tetrahydrofuran. In addition, 446 mg of 2-ethyl-3-methyl-4-hydroxy-6,7-difluoroquinoline (starting material 1) was suspended in 10 ml of tetrahydrofuran, and the suspension of starting material 1 was added dropwise to In the above sodium hydride suspension under ice cooling. The mixture was stir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com