Cephalosporins medicine intermediate crystal and method for preparing same
A technology for intermediates and crystals, applied in the field of pharmaceutical synthesis, can solve problems such as being unsuitable for commercial storage, and achieve the effects of no degradation of decomposition point, good crystallinity and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
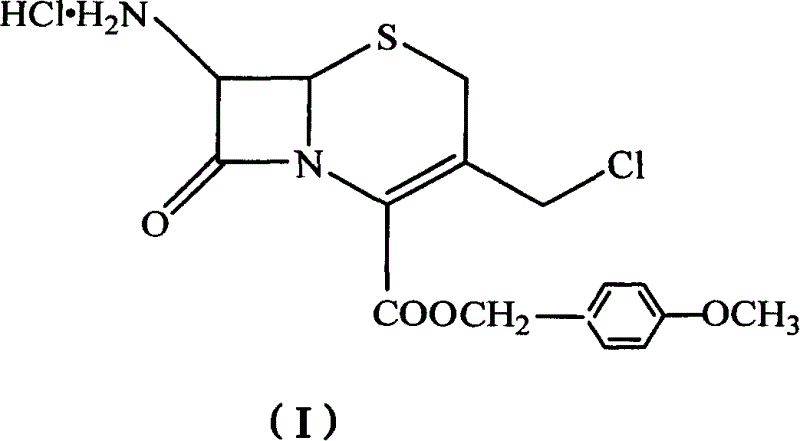
[0034] Preparation of the amorphous substance of compound (I):
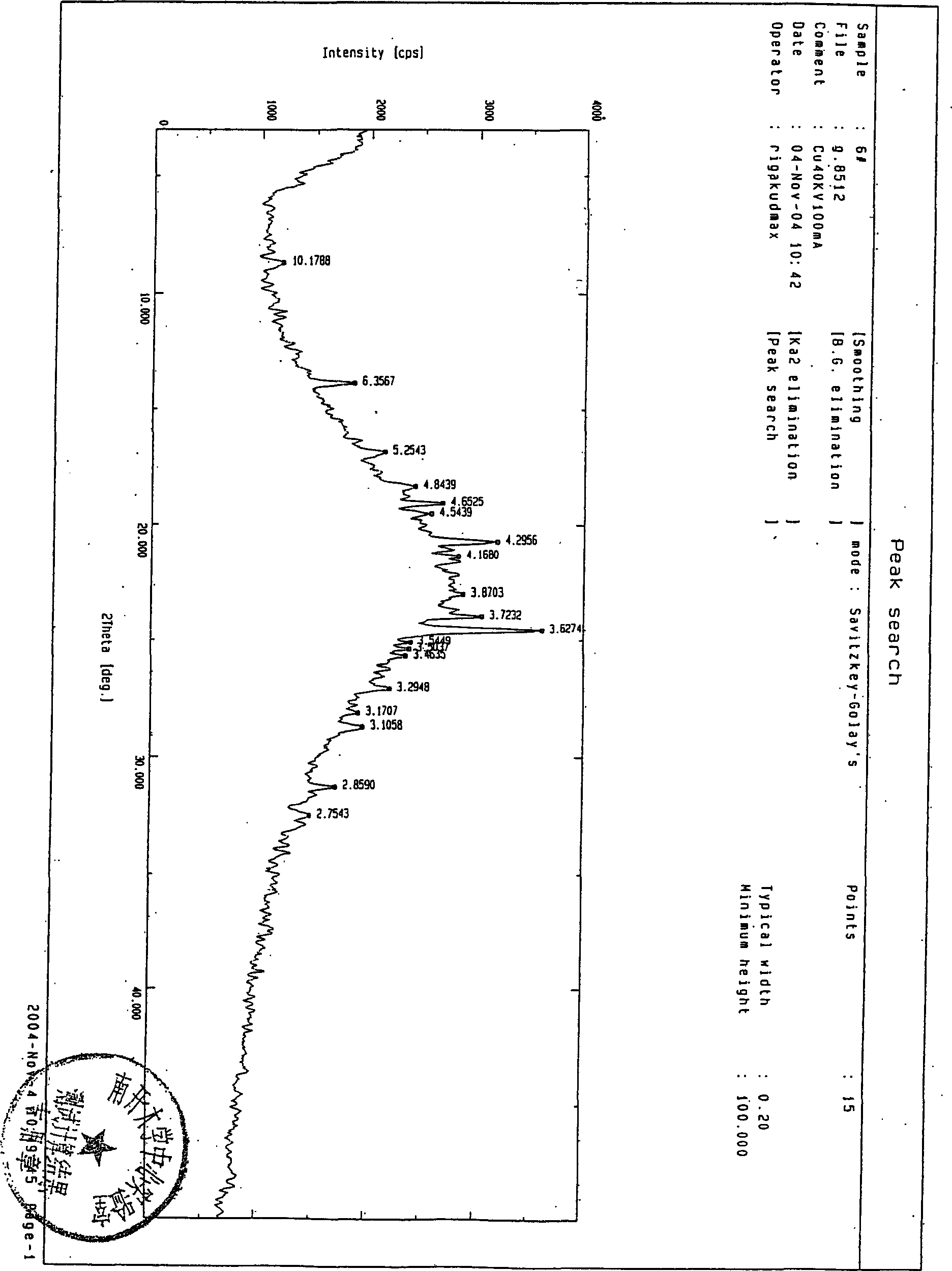
[0035] PCl 5 (20.7g, 0.099mol), dichloromethane (180mL) in a 500mL reaction flask, stirred and dissolved at room temperature, added dropwise 8mL of pyridine at 5°C, reacted for 30 minutes, added compound (II) (20g, 0.041mol), in At this temperature, react for 4 hours, then cool down to -25°C, add methanol, react for 2 hours, dropwise add normal water (180mL), and hydrolyze at 0°C for 1 hour to obtain 15.3g of an amorphous solid of compound (I). : 91%.
[0036] X-ray powder diffraction pattern see figure 2 , the measurement conditions are the same as those in Figure 1.
Embodiment 1
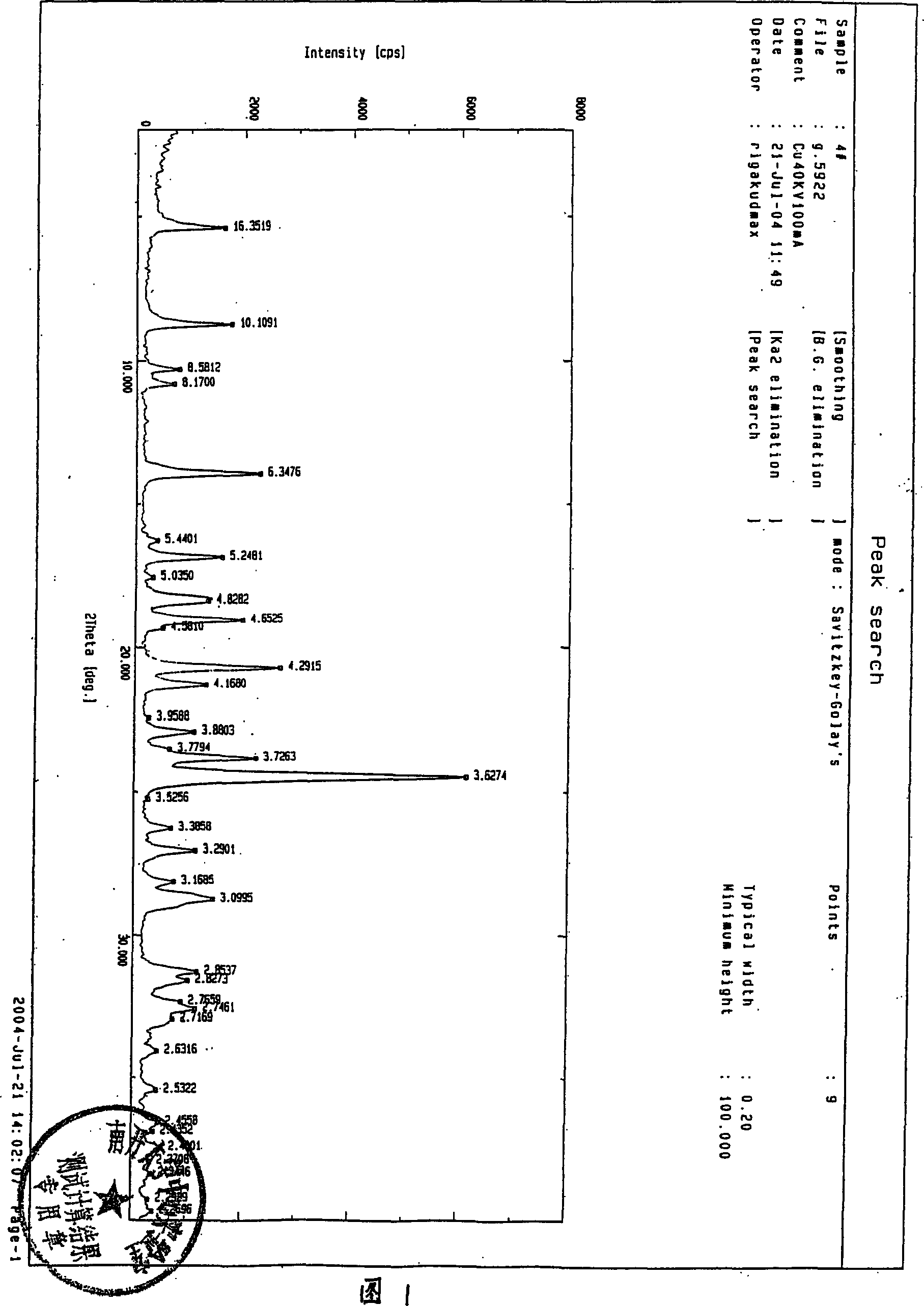
[0038] Take 50 g of the amorphous solid of Compound (I), add 150 ml of tetrahydrofuran, stir in an ice bath, add triethylamine dropwise at a temperature below 10°C until pH = 7.0, stir and react for 0.5 hours, then filter, add 600 ml of acetone to the filtrate, and add dropwise under stirring 20ml of concentrated HCl precipitated white crystals, filtered after cooling, and washed with an appropriate amount of acetone to obtain 45g of a white crystalline solid of compound (I), that is, 7-amino-3-chloromethyl-3-cephalosporanic acid-4-carboxylic acid p- Crystal of methoxybenzyl ester hydrochloride (I), yield 90%, melting point 148°C-150°C (decomposition point).
[0039] The infrared spectrum data are as follows:
[0040] IR (KCl, cm -1 ): 2899, 2835, 2765, 2602, 1786, 1723
[0041] Elemental Analysis: C 16 h 17 ClN 2 o 4 S·HCl
[0042] Found (%) C, 47.26; H, 4.41; N, 6.87; S 7.80; Cl 17.46
[0043] Theoretical value (%) C, 47.35; H, 4.44; N, 6.91; S 7.89; Cl 17.51
[004...
Embodiment 2
[0054] Take 50 g of the amorphous solid of Compound (I), add 300 ml of tetrahydrofuran, stir in an ice bath, add tert-butylamine dropwise at a temperature below 2°C until pH = 7.0, and filter after stirring for 0.5 hours, add 1,000 ml of butanone to the filtrate, and add concentrated 20ml of HCl precipitated white crystals, cooled and filtered, and washed with an appropriate amount of acetone to obtain 43g of white crystalline solids, which were the crystals of the compound shown in formula (I), with a yield of 86% and a melting point of 148°C-150°C (decomposition point).
[0055] The measurement conditions and measurement data of infrared spectrum, proton nuclear magnetic resonance spectrum, elemental analysis data and X-ray powder diffraction pattern are the same as embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com