6íõ-heterocycle substituted spirooxazine photochromic compound and method for preparing same
A technology of photochromism and compounds, applied in the direction of chemical instruments and methods, color-changing fluorescent materials, etc., to achieve the effect of less dosage, bright colors and good fatigue resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The first step, synthesis of nitrosonaphthol
[0029] Dissolve 5.7g (0.04mol) of 2-naphthol in 68mL of hot water containing 1.6g (0.04mol) of NaOH, place in an ice-salt bath, cool to 0°C, add 2.8g (0.04mol) of sodium nitrite, Add 9.5 mL of sulfuric acid with a concentration of 5.6 mol / mL dropwise under stirring, keeping the temperature at about 0°C. After the addition, continue stirring at 0°C for 1 hour, and then add a small amount of (NH 4 ) 2 SO 4 to remove excess sodium nitrite. Suction filtration, the solid was fully washed with water, and dried to obtain a yellow solid, which was recrystallized from petroleum ether (60-90° C.) to obtain 5.4 g of reddish-brown needle-like crystals with a yield of 79%, m.p.106-107° C. (lit. 106°C).
[0030]
[0031] The second step, synthesis of 5-chloro-1,3,3-trimethyl-2-methylidene indoline
[0032] Dissolve 8.39g (0.025mol) of 5-chloro-1,2,3,3-tetramethylindoline iodide in 25mL of water, add 8mL of aqueous solution contai...
Embodiment 2
[0041] The first step, synthesis of 1,3,3-trimethyl-2-methyleneindoline
[0042] Dissolve 7.5g (0.025mol) of 1,2,3,3-tetramethylindoline iodide in 25mL of water, add 8mL of aqueous solution containing 1.6g (0.04mol) of NaOH, stir at room temperature for 30 minutes, and wash with chloroform extraction, the organic phase was washed with water, and washed with anhydrous MgSO 4 After drying, the solvent was distilled off to obtain 3.9 g of a colorless liquid with a yield of 90%, which would rapidly turn red when placed in air.
[0043]
[0044] The second step, synthesis of 1,3,3-trimethyl-6'-decahydroisoquinoline-spiroindoline-2,3'-[3H]naphtho[2,1-b][1,4 ] Synthesis of oxazines
[0045]
[0046] 1.73g (0.01mol) of nitrosonaphthol, 2.78g (0.02mol) of decahydroisoquinoline, 50mL of trichlorethylene as a solvent, heated to reflux for 30 minutes, slowly added dropwise 1.73g of indoline, and continued to reflux for 3 -4 hours, spin-dried, and performed column chromatography w...
Embodiment 3
[0050] The first step, synthesis of 5-chloro-1,3,3-trimethyl-6′-hexamethyleneimine-spiroindoline-2,3′[3H]naphtho[2,1-b] [1,4]oxazine
[0051]
[0052] 2g (0.011mol) of nitrosonaphthol and 1.13mL of hexamethyleneimine were introduced into N in 30mL of anhydrous methanol 2 , heated to reflux for 30 minutes, added dropwise 1.3g (0.006mol) 5-chloro-1,3,3-trimethyl-2-methyleneindoline and continued to heat and reflux overnight to obtain 0.23g of photochromic product, producing The rate is 8.42%.
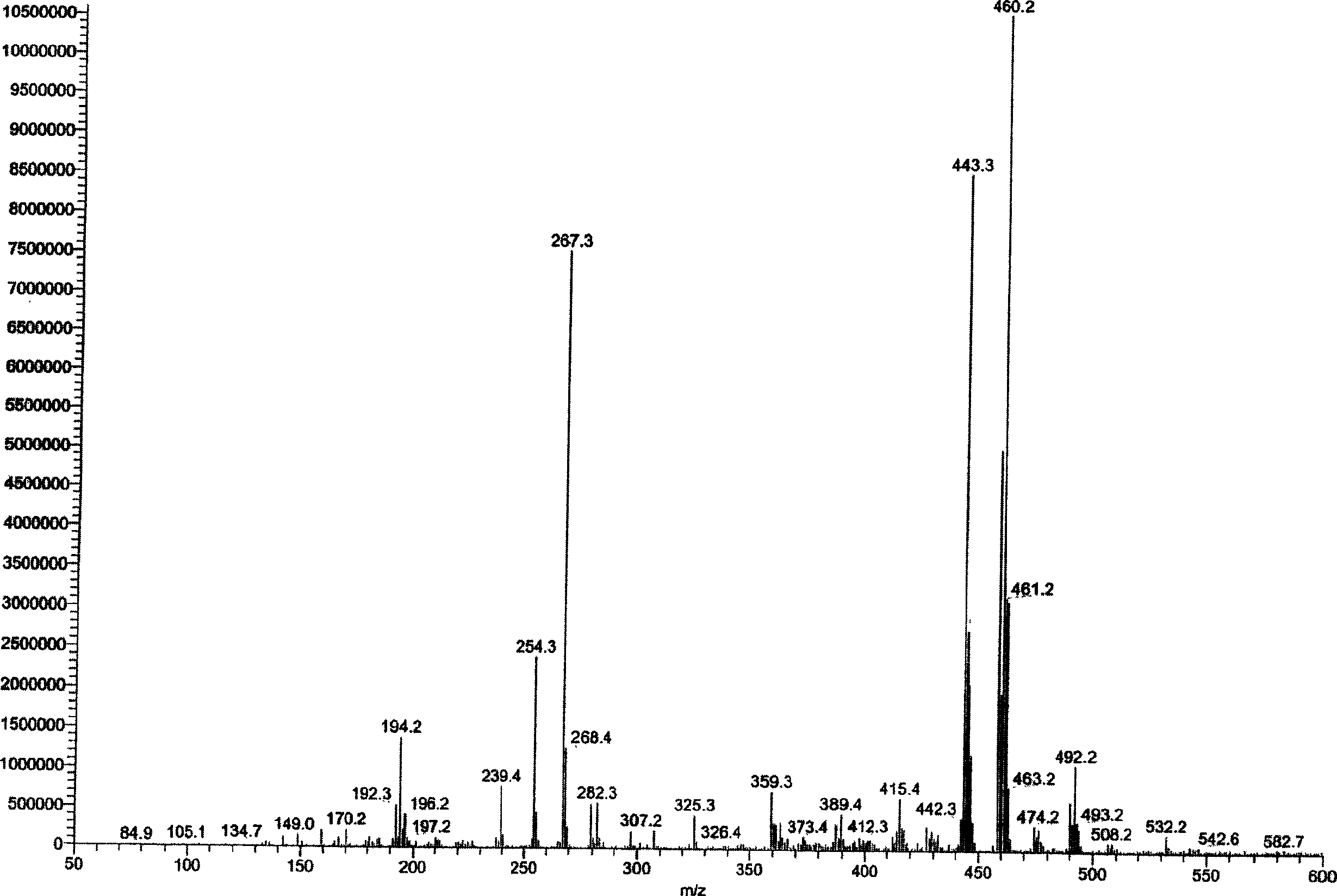
[0053] image 3 Example 3 Synthetic Compound Mass Spectrum.
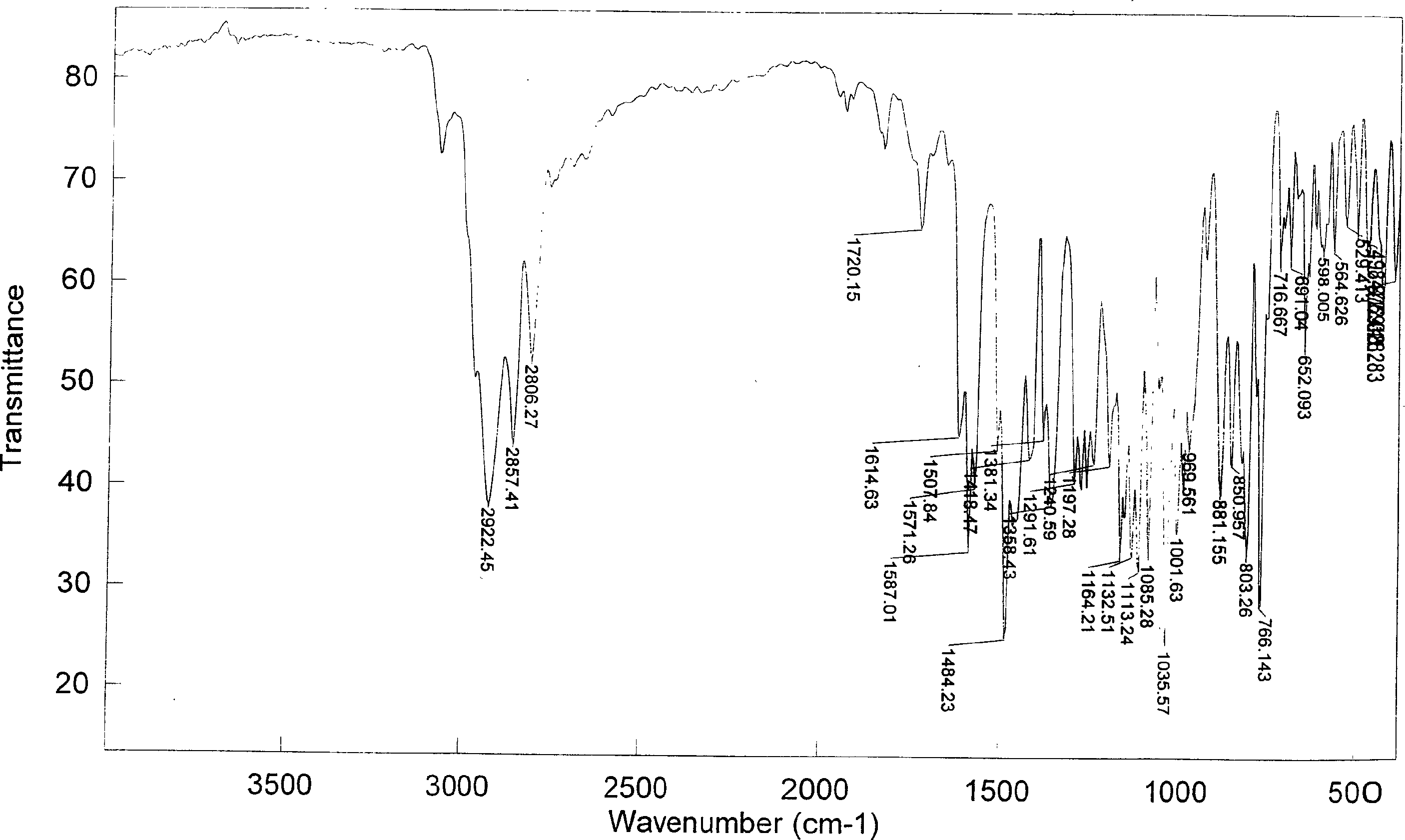
[0054] The second step, detection:
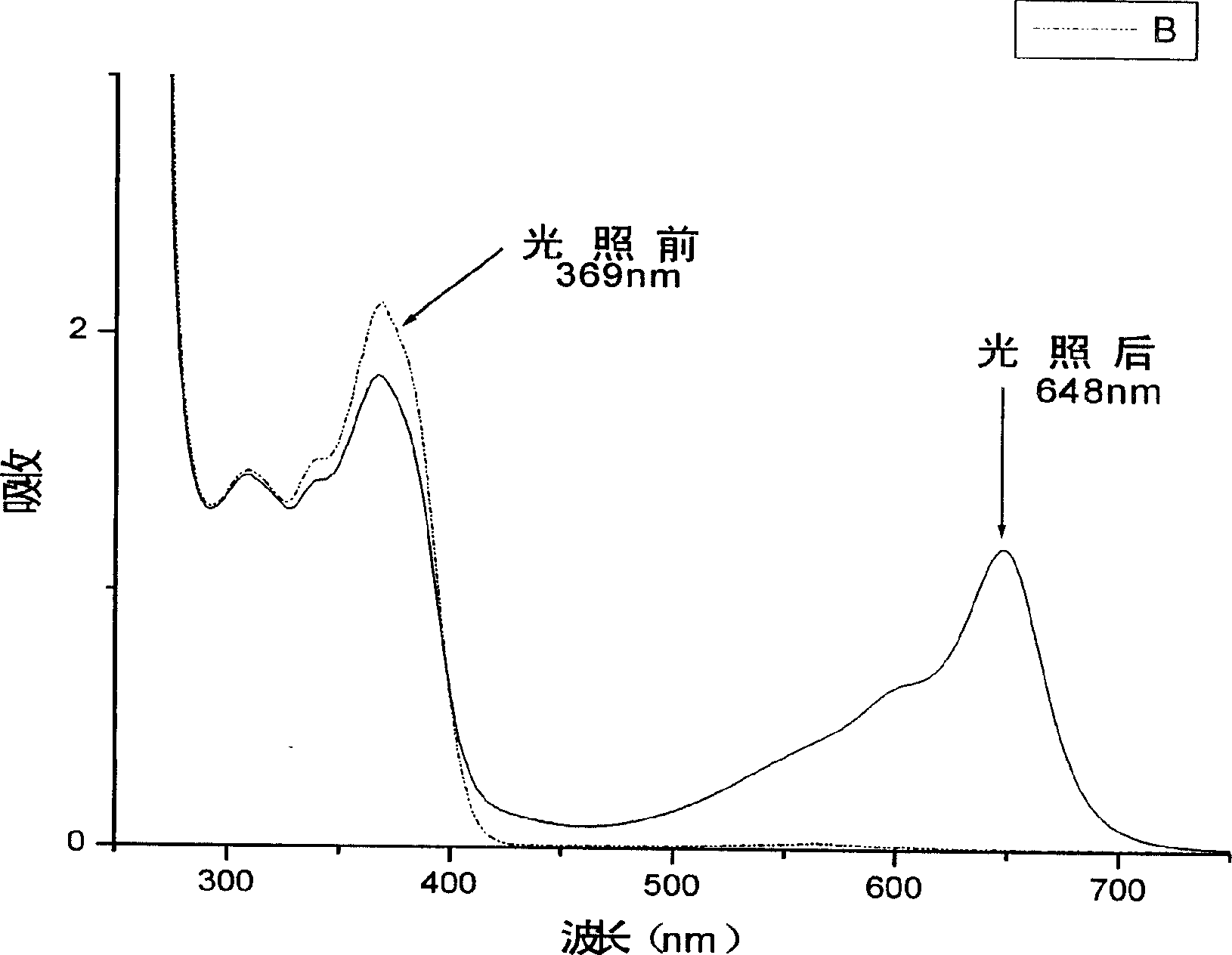
[0055]Photochromic substances can be observed to change the color of the solution to blue-purple under the irradiation of ultraviolet light or sunlight. At the same time, the maximum absorption peak of ultraviolet absorption wavelength can be observed at 589nm after irradiation with ultraviolet spectrophotometer -1 place.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com