Antigenic determinant polypeptide of human tumor-testis antigen HCA587 and use thereof
An antigenic determinant, testis antigen technology, applied in the direction of antineoplastic drugs, animal/human peptides, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1. Gene discovery process of CT antigen HCA587
[0027] In order to screen abnormally expressed CT antigens in primary hepatocellular carcinoma, we used the cDNA synthesis Kit, Uni-ZAP XR vector and Gigapack IIIGOLD packaging extract kit from Stratagene to select tissue markers from patients with hepatocellular carcinoma for SEREX analysis. filter.
[0028] First, extract total RNA from liver cancer and paracancerous tissues according to the operating instructions of the TRIzol kit (Gibco Company). The steps include: tissue homogenate, extraction, RNA precipitation, washing and dissolving. Ultrapure water dissolves the precipitate. The OD260 and OD280 values of the samples were measured by ultraviolet spectrophotometer, and the RNA quality (the ratio of 28S and 18S rRNA) was detected by formaldehyde denaturing gel electrophoresis.
[0029] The PolyATract mRNA Isolation Kit (Promega, Madison, WI) from Promega Company was used to isolate mRNA. oligo(dT) bindi...
Embodiment 2
[0036] Example 2. Expression of HCA587 recombinant protein and its distribution in tumor tissue
[0037] (1) Expression of recombinant pQE30-HCA587 protein
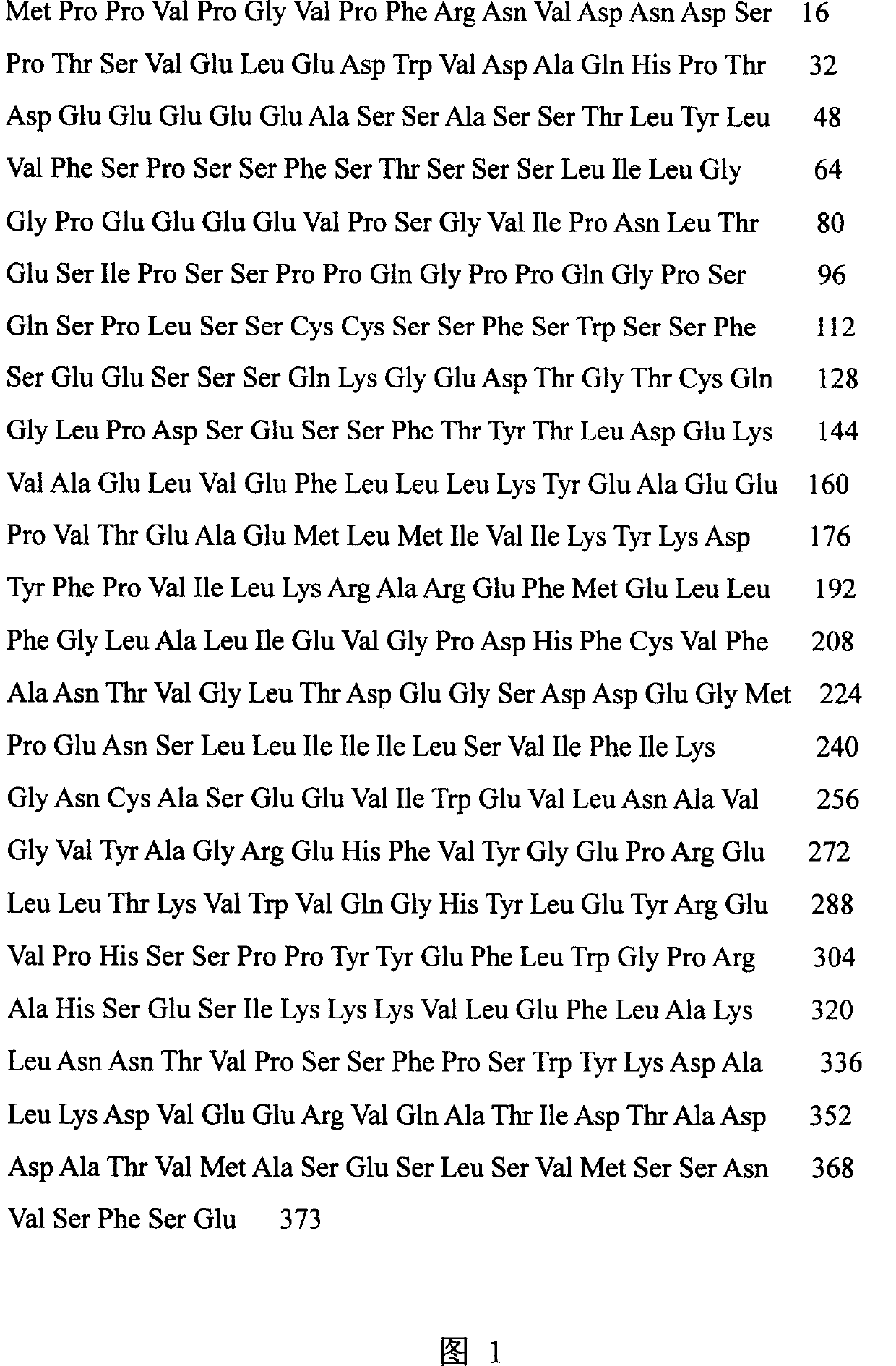
[0038] Design specific primers according to the nucleotide sequence of HCA587, synthesize BamHI and HindIII restriction sites at the 5' ends of the upstream and downstream primers, P1: 5'ATC GGATCC CCTCCCGTTCCAGGCGT3',P2:5'ACT AAGCTT TCACTCAGAAAAGGAGAC3', using the cDNA of normal testis tissue as a template, the full-length ORF of HCA587 was amplified by PCR, and after the PCR product was connected with the pGEM-Teasy vector, it was transformed into DH5a, and the positive clones containing the correct insertion of HCA587 were selected, and double-expressed with BamHI and HindIII, respectively. Digest the HCA587-pGEMT-easy plasmid and the pQE-30 vector, recover the HCA587 and pQE-30 fragments from the gel after electrophoresis, connect with T4 ligase overnight, transform XL-Blue competent cells, and extract the recombina...
Embodiment 3
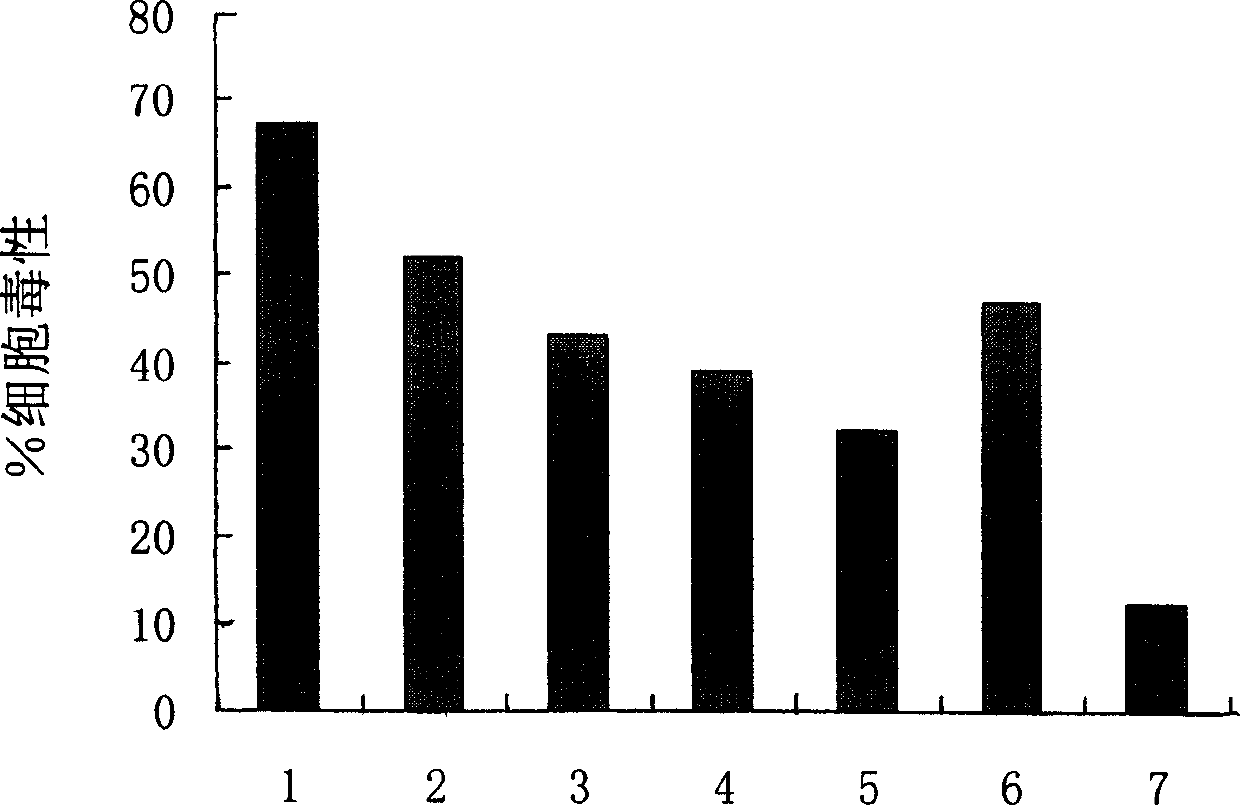
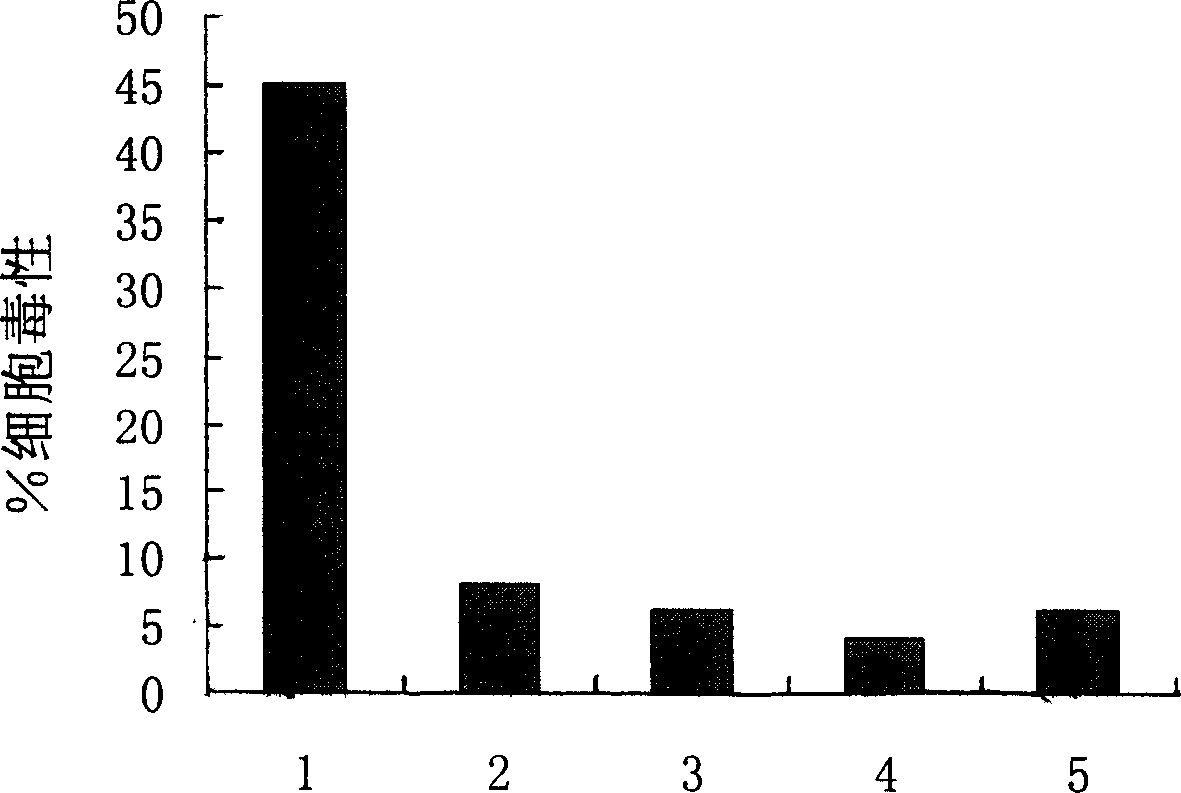
[0060] Example 3. T2 binding assay
[0061]To analyze HCA587 to induce the body to produce an immune response, it is first required that the protein is processed by APC cells, and the corresponding polypeptide is combined with its HLA molecule and presented to the cell surface. Therefore, we first used the model invented by Dr.Kenneth Parker (Parker, K.C., et al.1994.J.Immunol.152:163) to predict the polypeptide that can bind to HLA-A2 in the HCA587 protein sequence (web site: http: / / www-bimas.cit.nih.gov / molbio / hla_bind / ), combined with other HLA-A2 predicted binding peptide software, screened out 9 more likely antigenic peptides bound to HCA587 and HLA-A2, and synthesized all of them with a peptide synthesizer in vitro The predicted polypeptide (see Table 2) was purified by HPLC with a purity of 99%, and then the cell line CEMX721.174.T2 (T2 for short) was used for HLA-A2 binding test. Because T2 cells only express HLA-A2 molecules, but cannot present endogenous polypeptid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com