Human rapid contraction of skeletal muscle troponin I production and activity detection method
A troponin and production method technology, applied in the field of genetic engineering biology, can solve the problems of high price of chromatography medium, large errors in biological activity determination methods and quality control indicators, and difficulty in standardization, and achieve obvious angiogenesis and inhibition of vascular endothelium. Cell proliferation, inhibition of angiogenesis and inhibition of vascular endothelial cell proliferation, overcoming the effect of large errors
Inactive Publication Date: 2005-01-26
NANJING UNIV
View PDF1 Cites 11 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
The purification process of Zhou Yiqun et al. is relatively simple, but because the final purified product is recombinant human troponin I-His6, that is, 6 consecutive His are added to the end of human troponin IC, and recombinant human troponin I-His6 His6 has become a new protein, and its properties such as safety in vivo will need to be re-evaluated
In the process of Moses et al., the chromatographic medium Progel-TSKG3000SWXL used is expensive and difficult to purchase in China
Moreover, both methods of viability measurement rely on capillary endothelial cell proliferation experiments and various animal model experiments, which are time-consuming, expensive, and have large data fluctuations and poor repeatability.
However, the vascular endothelial cells used currently have no cell lines and are all primary cells. The primary vascular endothelial cells prepared in different batches are different due to different sources of materials and differences between experiments. Therefore, capillary endothelial cells were used As a bioactivity assay and quality control indicator of recombinant human troponin I, cell proliferation experiment undoubtedly has problems such as large errors and difficulty in standardization
However, it is more time-consuming, labor-intensive, more error-prone and difficult to standardize by using animal model experiments as routine bioactivity assay methods and quality control indicators.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
[0031] 1. Using human fast-twitch skeletal muscle troponin I cDNA as a template, synthesize the following PCR primers:
[0032]Primer 1: 5'-CTCACCATGGAGATGAGGAGAAGC-3';
[0033] Primer 2: 5'-GCCTCGAGTGGCCTAGGACTCGGAC-3';
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
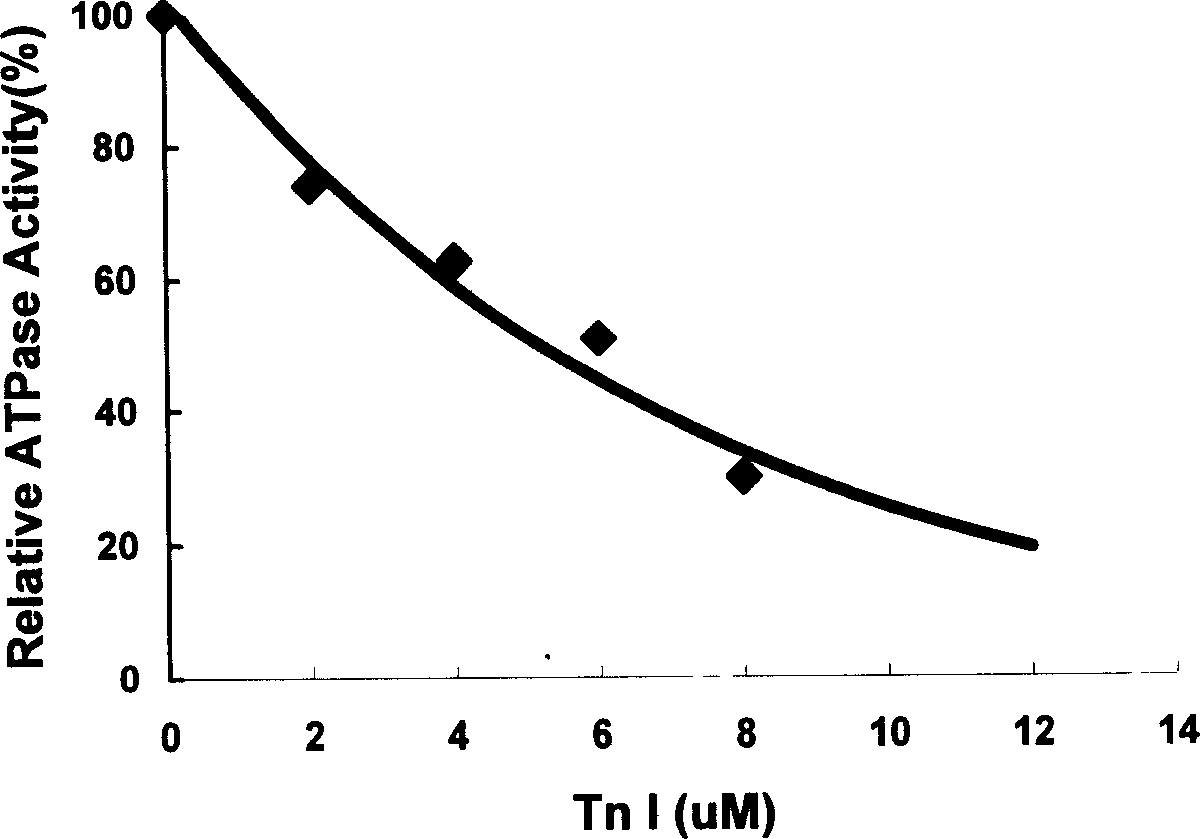
The invention belongs to gene engineering field. The invention establish a production method of human skeletal muscle troponin I for quick contract: recombing DNA to construct expression plasmid, refining expression products with inclusion bodies, cation exchange resin column chromatography, cation exchange resin column chromatography, renaturing inclusion bodies, at last purifying and obtaining recombinant human troponin I for suppressing blood vessel endothelial cell propagation and angiogenesis. The invention also establishes a method for determing the biological activity index of troponin I by suppressing Acto-S1 Mg-ATPase activity. The troponin I can prepare drugs of the tumor and angiogenesis related disease
Description
1. Technical field: [0001] The invention belongs to the field of genetic engineering biotechnology. 2. Background technology: [0002] Troponin includes 3 subunits: Troponin C, I, and T. Troponin I is a 182-amino acid protein that can inhibit the ATPase activity of actomyosin. In 1999, Moses et al. (Proc.Natl.Acad.Sci.USA, 1999, 96:2645-2650) first discovered a protein in cartilage that inhibits bFGF (basic fibroblast growth factor)-stimulated capillary endothelium Cell proliferation activity and demonstrated that the protein is human fast-twitch skeletal muscle troponin I. Further studies have shown that: human fast-twitch skeletal muscle troponin I has the biological activity of inhibiting angiogenesis and tumor metastasis (Proc.Natl.Acad.Sci.USA, 1999,96:2645-2650), and its mechanism of action may be through Competing with bFGF for bFGF receptors (Microvascular Research, 2002, 63: 41-49). Since troponin I has the biological activity of inhibiting angiogenesis and tumo...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K38/17A61P35/00C07K1/18C12N15/12C12N15/70G01N33/68
Inventor 华子春颜明
Owner NANJING UNIV
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Patsnap Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com