Method for synthesizing Lomerizine Hydrochlortde
A technology of lomerizine hydrochloride and its synthesis method, which is applied in the direction of organic chemistry, etc., and can solve the problems of low industrial production value, low yield of lomerizine hydrochloride synthesis technology, and difficulty in obtaining medicinal crystal forms of lomerizine hydrochloride To achieve the effect of shortening treatment time, reasonable treatment, and relieving pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
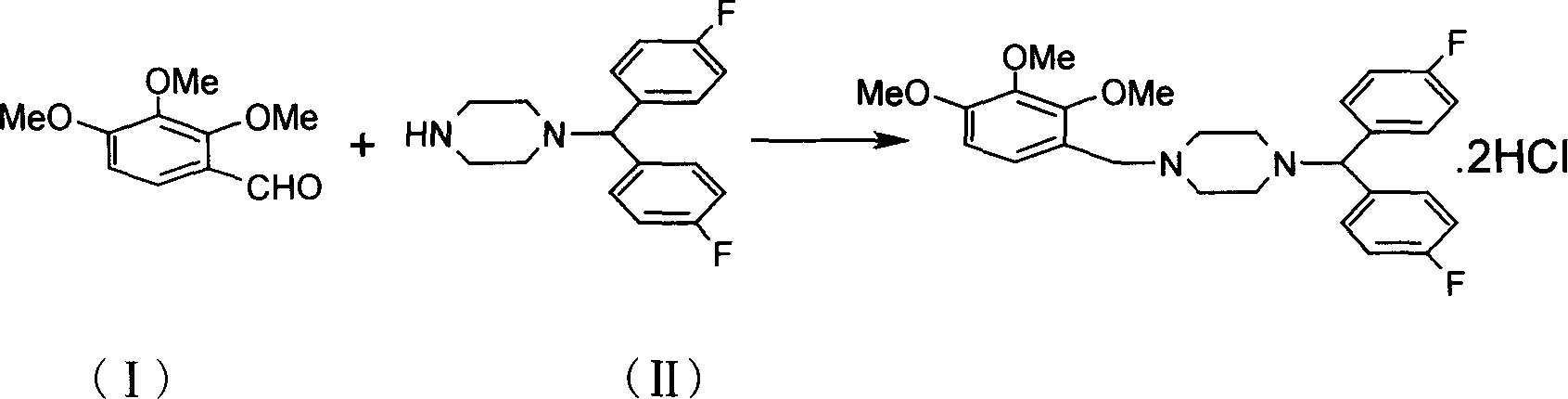
[0021] Put 1020g of 2,3,4-trimethoxybenzaldehyde and 1500g of bis(4-fluorophenyl)methylpiperazine into the reaction flask, and slowly melt it in an oil bath at 100°C. After the melting is complete, slowly add 260ml of formic acid dropwise, After dripping, continue to stir and react at this temperature for 1 hour, be cooled to room temperature, add 960ml concentrated hydrochloric acid (37%) and 9600ml absolute ethanol and stir and reflux for 1 hour, then add gac 60g, continue to stir and decolorize 0.5 hour. After filtration, the filtrate was slowly cooled and crystallized to obtain 2507.4 g of white crystals, with a yield of 90.02%, mp: 202°C (document mp: 204°C). The crude product of lomerizine hydrochloride can still be obtained after cooling the mother liquor, which can increase the yield.
[0022] Get lomerizine hydrochloride crude product 1950.3g, add acetonitrile-methanol (80%-20%) mixed solution 7000ml, heat under stirring for 1 hour, cool and filter to obtain white crys...
Embodiment 2
[0024] Put 1020g of 2,3,4-trimethoxybenzaldehyde and 3750g of bis(4-fluorophenyl)methylpiperazine into the reaction flask, and slowly melt it in an oil bath at 100°C. After the melting is complete, slowly add 260ml of formic acid dropwise, After dripping, continue to stir and react at this temperature for 3 hours, be cooled to room temperature, add 960ml concentrated hydrochloric acid (37%) and 9600ml absolute ethanol and stir and reflux for 5 hours, then add gac 90g, continue to stir and decolorize 1 hour. After filtration, the filtrate was slowly cooled and crystallized to obtain 2548.6 g of white crystals, with a yield of 91.5%, mp: 202° C. (document mp: 204° C.).
[0025] Take 1950.3 g of lomerizine hydrochloride crude product, add acetonitrile-ethanol (80%-20%) mixed solution 7000 ml, heat with stirring for 1 hour, cool and filter to obtain white crystals, and dry to obtain fine product 1057.7 g. Yield 54.23%, mp: 207.8-208.9°C.
[0026] Acetonitrile-methanol ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com