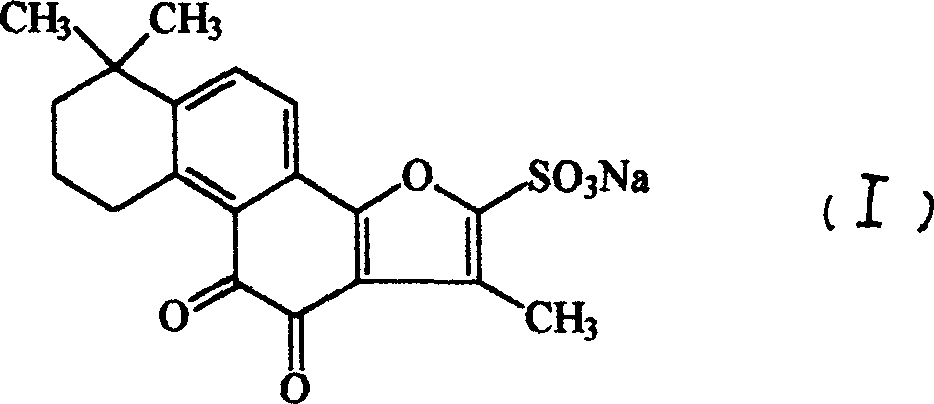
Tanshinone II A sodium sulfonate powder injection agent and its preparation method
A technology of sodium sulfonate and tanshinone, applied in powder delivery, cardiovascular system diseases, drug combination and other directions, can solve the problems of affecting application, difficulty in storage, reducing stability and quality of preparations, etc., to improve drug quality and stability , Increase the effect of drug stability, clarity and stability improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Weigh 10g sodium tanshinone IIA sulfonate, 10g mannitol, 0.1g vitamin C and add 800ml of water for injection to dissolve, adjust the pH value to 4.5, filter through a 0.45μm microporous membrane; place the filtrate at 0°C for 6 hours, 0.1 μm microporous membrane filtration, diluted with water for injection to 1000ml, filled (2ml / bottle), freeze-dried, sterilized by radiation, and packaged.
Embodiment 2
[0045] Weigh 10g sodium tanshinone IIA sulfonate, 10g mannitol, 0.1g vitamin C and add 100ml water for injection to dissolve, adjust the pH value to 5, filter through a 0.45μm microporous membrane; place the filtrate at 1°C for 10 hours, 0.1 μm microporous membrane filtration, diluted with water for injection to 200ml, spray-dried, potted (40mg / bottle), sterilized by radiation, and packaged.
Embodiment 3
[0047] Weigh 10g of sodium tanshinone IIA sulfonate and 10g of mannitol, add 100ml of water for injection to dissolve, adjust the pH value to 5.5, filter through a 0.45μm microporous membrane; place the filtrate at 2°C for 12 hours, filter through a 0.1μm microporous membrane Membrane filtration, diluted with water for injection to 200ml, spray-dried, potted (20mg / bottle), sterilized by radiation, and packed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com