Gene transfer-mediated angiogenesis therapy
A vascular, transgenic technology, applied in the field of gene therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
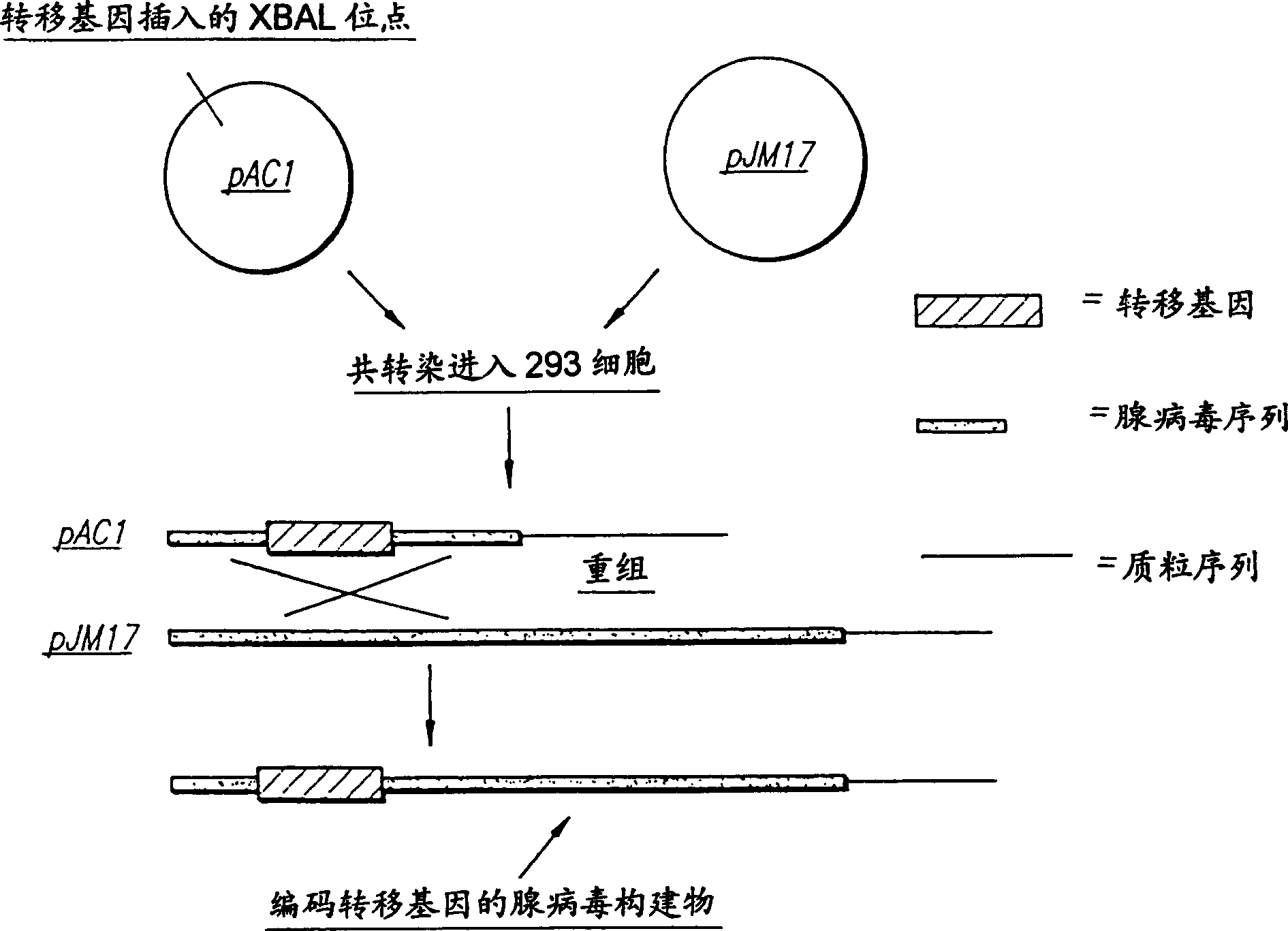
[0121] Example 1: Adenovirus Constructs
[0122] A helper-independent replication-deficient human adenovirus 5 system was used with the target genes LacZ and FGF-5. The full-length cDNA of human FGF-5 is a 1.1 kb ECOR1 fragment from plasmid pLTR122E (Zhen, et al, Mol. Cell. Biol., 8:3487, 1988), which includes a 981 bp gene open reading frame, which was cloned into Multiple cloning site for plasmid ACCMVPLPA containing the CMV promoter and SV40 polyadenylation signal and part of the flanking adenovirus sequence from which the E1A and E1B genes (essential for viral replication) have been deleted. This plasmid was co-transfected (lipofection) into 293 cells with the JM17 plasmid, which contains the entire genome of human adenovirus 5 and an additional 4.3 kb insert, which makes pJM17 too large to be packaged. Homologous salvage recombination forms an adenoviral vector containing the transgene lacking the E1A / E1B sequence. Although these recombinants were inca...
Embodiment 2
[0123] Example 2: Adult murine cardiomyocytes in cell culture
[0124] Adult rat cardiomyocytes can be prepared by Langendorf perfusion according to standard methods using perfusate containing collagenase. The rod-shaped cells were cultured on a laminin-coated plate for 24 hours, and infected with the adenovirus encoding β-galactosidase obtained in Example 1 above at a multiplictity of 1:1. Glutaraldehyde fixation and incubation with X-gal. In good agreement with the above results, 70-90% of adult rat cardiomyocytes were infected with recombinant adenovirus and expressed β-galactosidase, and no cytotoxicity was observed when the multiplicity of infection was 1-2:1.
Embodiment 3
[0125] Example 3: In vivo porcine myocardium
[0126] According to the method of Example 1, the adenovirus vector encoding β-galactosidase obtained in Example 1 was propagated in compatible 293 cells, and purified by CsCl gradient ultracentrifugation until the final virus titer was 1.5×10 10 virus particles. Thoracotomy was performed on an anesthetized, ventilated 40 kg pig, a 26-gauge butterfly needle was inserted into the middle of the left anterior descending coronary artery (LAD), and a 2 ml volume of vehicle (1.5 ×10 10 virions), the chest was sutured to allow the animal to recover. Animals were sacrificed on the fourth day after vehicle injection, hearts were fixed with glutaraldehyde, incubated with X-gal for 16.5 hours after sectioning, and tissues were counterstained with eosin after embedding and sectioning.
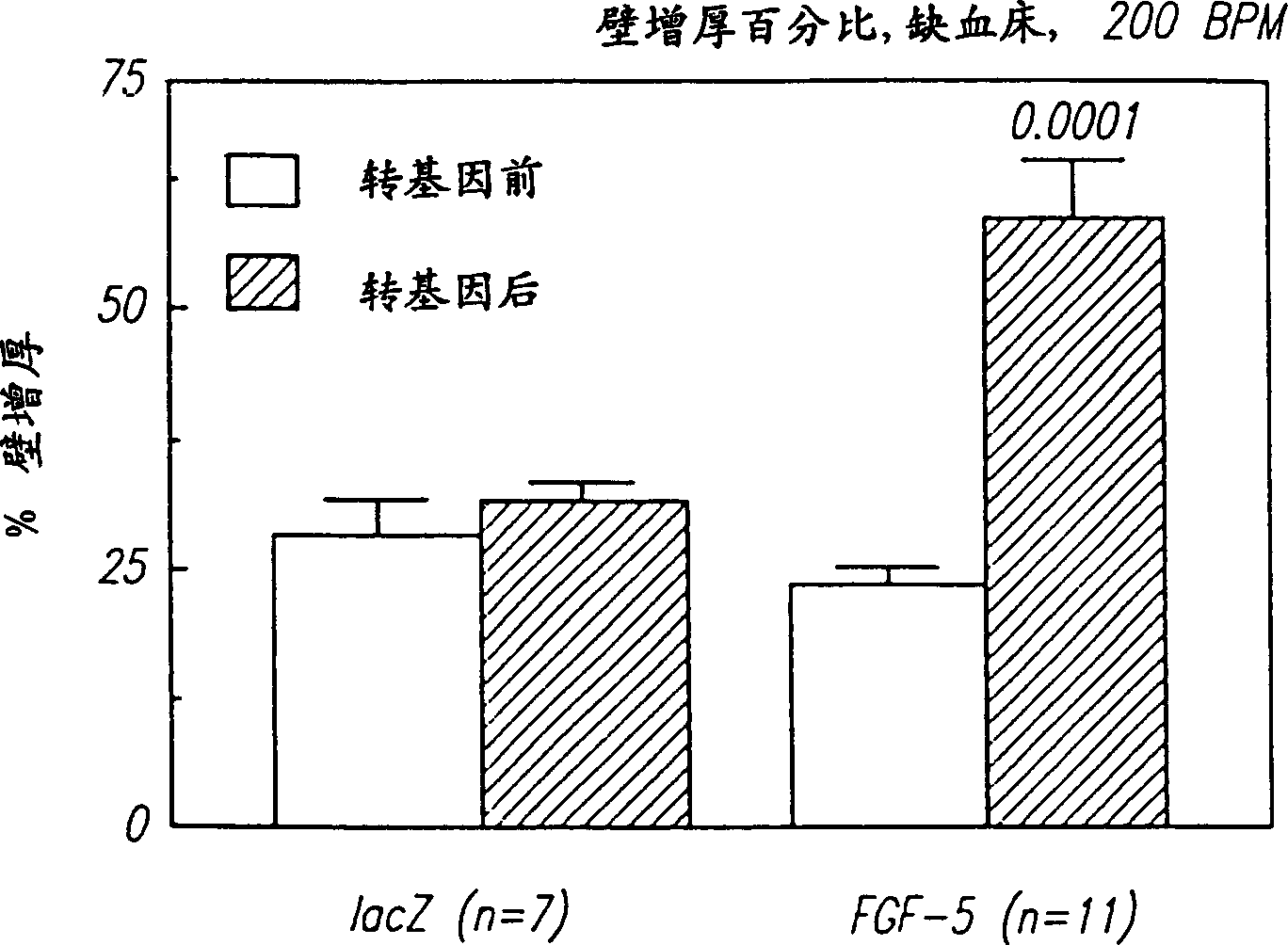
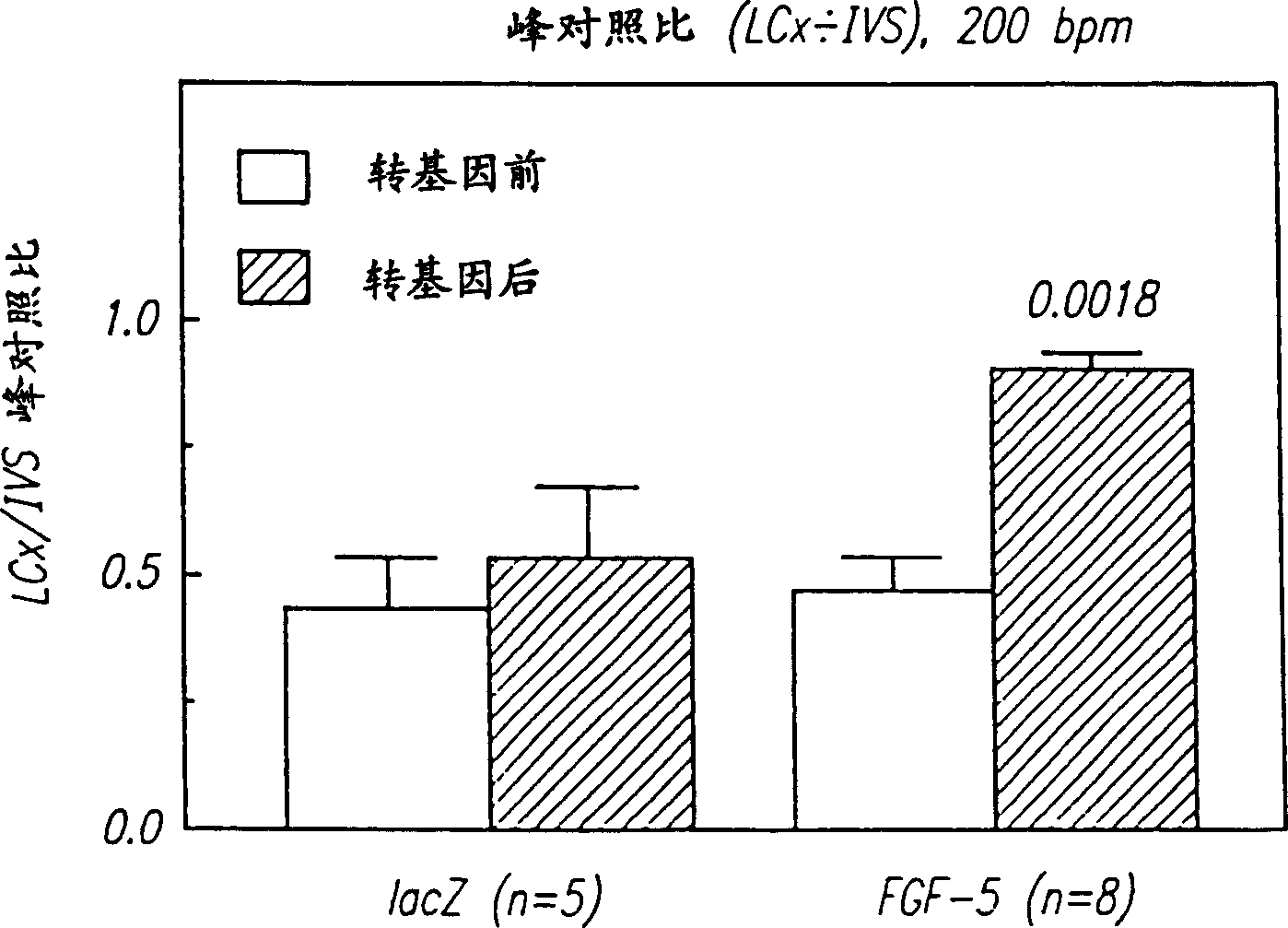
[0127] Microscopic observation of tissue sections (full-thickness tissue sections of the LAD bed 96 hours after intracoronary injection of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com