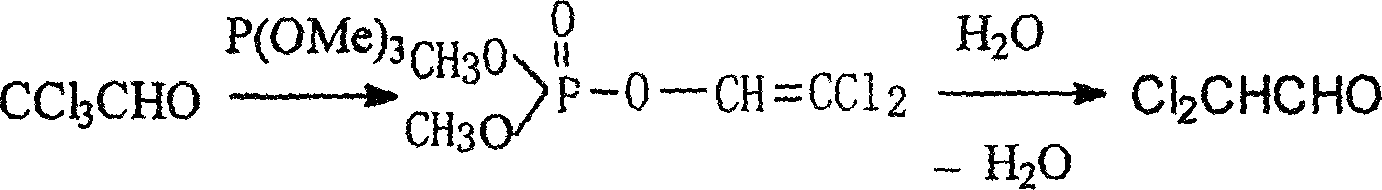Method for preparing dichloro acetaldehyde from hydrated chloral
A technology of hydrated chloral and anhydrous chloral, which is applied to the preparation of carbon-based compounds, the preparation of organic compounds, chemical instruments and methods, etc. It can solve the problems of difficult industrial production, high production costs, and affecting reactions, etc. problems, achieve the effects of reducing concentrated sulfuric acid pollution, reducing equipment investment, and increasing operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0015] Add 166 grams of chloral hydrate and 165 milliliters of benzene (volume ratio is about 1:1) into a 1000 milliliter three-necked flask, and connect an electric stirrer, a thermometer and a water separator respectively. Adjust the temperature to about 110°C and heat for about 1 hour and 30 minutes. When the amount of water in the water separator no longer increases, remove the water separator and use an ice-water bath instead, add 160 ml of trimethyl phosphite dropwise from the constant pressure dropping funnel, and control the dropping rate so that the reaction temperature does not exceed 30°C. The dropwise addition time is about 1 hour and 10 minutes. The reaction was continued for 15 minutes. After the reaction is completed, the solvent benzene and other impurities below 100°C are distilled off. Cool slightly, add 30 ml of distilled water and a small amount of concentrated sulfuric acid, distill under normal pressure, collect fractions at 89.0-91.0°C, and obtain 104....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com