Photoepolymerization initiater
A technology of photopolymerization initiators and compounds, applied in dental preparations, dental prostheses, compression mold cups, etc., can solve the problem of decreased polymerization activity, and achieve the effects of high mechanical strength, high polymerization activity, and good storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0106]The present invention will be described more specifically by means of working examples below, but the present invention is not limited by the following manner.
[0107] Abbreviations for compounds used in the following Examples and Comparative Examples.
[0108] (1) Abbreviation
[0109] (A) α-diketone
[0110] CQ: Camphorquinone
[0111] (B1) Aliphatic amine compound
[0112] (B1-1) A compound having two to three saturated aliphatic groups substituted with electron-attracting groups.
[0113] TEOA: Triethanolamine
[0114] EDEOA: N-Ethyldiethanolamine
[0115] MDEOA: N-Methyldiethanolamine
[0116] TAA: Triallylamine
[0117] (B1-2) A compound having only one saturated aliphatic group substituted with an electron-attracting group.
[0118] DMEM: N,N-dimethylaminoethyl methacrylate
[0119] DEEOA: N,N-Diethylethanolamine
[0120] (B1-3) A compound not containing a saturated aliphatic group substituted with an electron-attracting group.
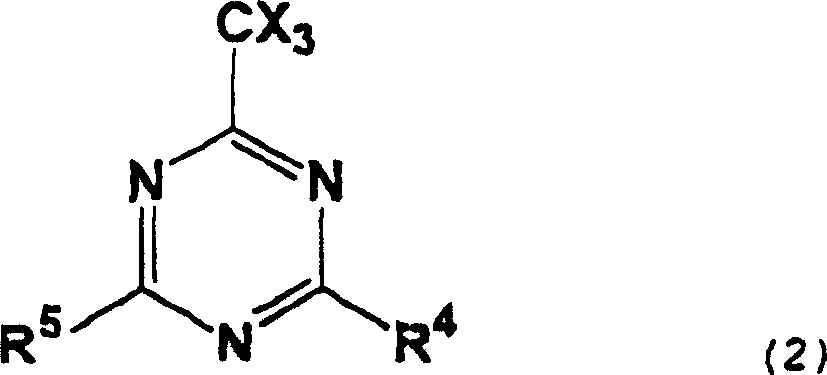
[0121] TEA: Triethylam...
Embodiment 1
[0190] As polymerizable monomers, D2.6E (70 parts by mass), 3G (25 parts by mass) and UDMA (5 parts by mass) were used, and a polymerization initiator, namely, CQ (0.3 parts by mass), DMPT (0.25 parts by mass) was added thereto. parts), DMEM (0.25 parts by mass) and TCT (0.4 parts by mass). The mixture was dissolved in the dark to obtain a homogeneous solution. The curing properties and Vickers hardness of the solutions were evaluated by using three dental irradiators (LM, A95, TP). The results are shown in Table 1.
Embodiment 2 and 3
[0205] Embodiment 2 and 3, comparative examples 3 to 6
[0206] Solutions were prepared in the same manner as in Example 1, except that the composition of the polymerization initiator was changed as shown in Table 2, and evaluated. Curing performance was evaluated with a halogen lamp (TP). The results are shown in Table 2.
[0207] Table 2
[0208] Photopolymerization initiator / mass parts TP curing properties hardness
[0209] CO DMPT DMEM TCT Ph2IPF6 5 10 15 LM A95 TP
[0210] sec sec sec.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com