N-end fusion protein PC-1-e2 of human multicapsular protein-1
A polycystin and fusion protein technology, applied in the field of medical molecular bioengineering, can solve the problems of high cost, short half-life, and limited intervention effect, and achieve low cost and long-lasting inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
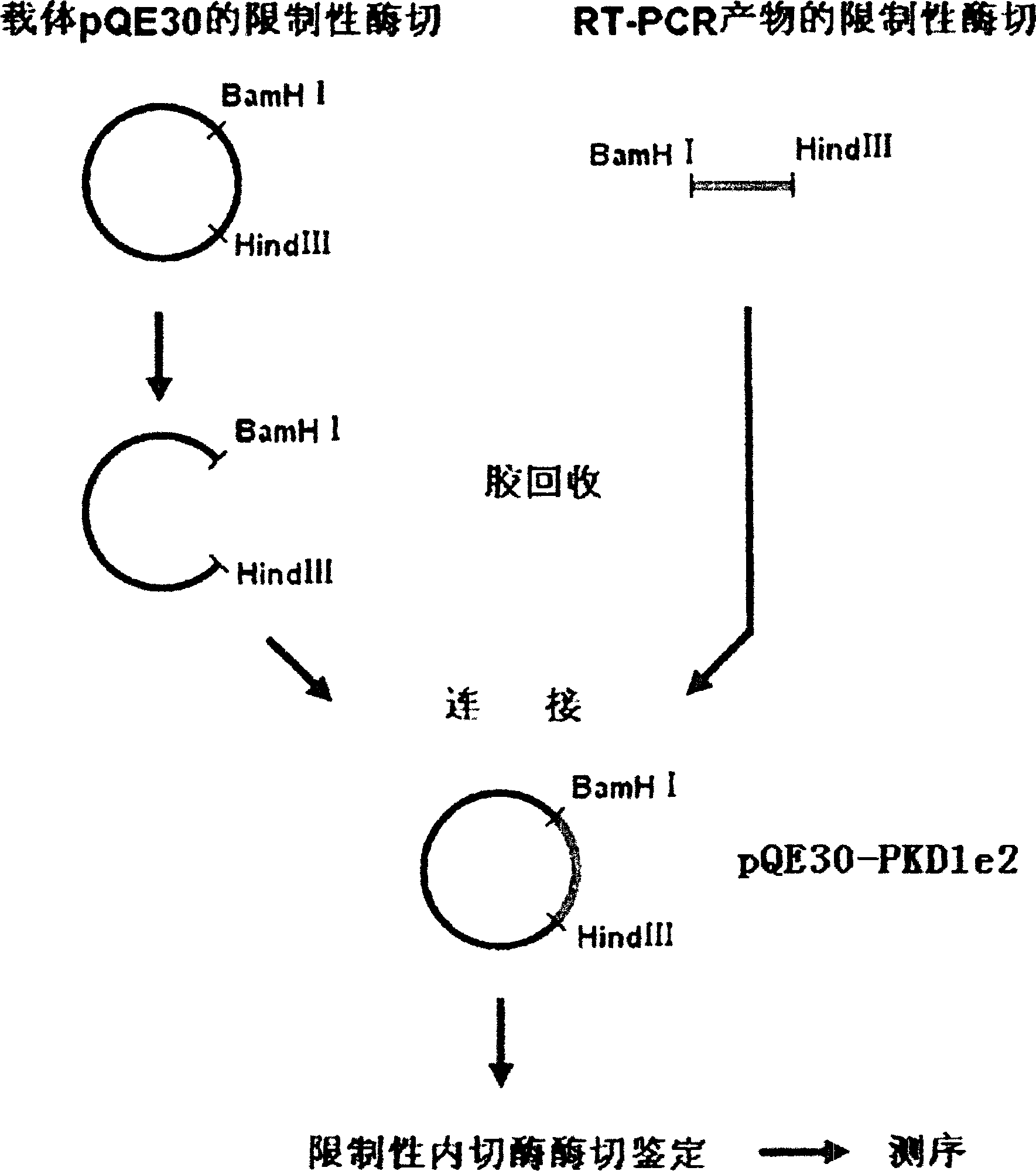
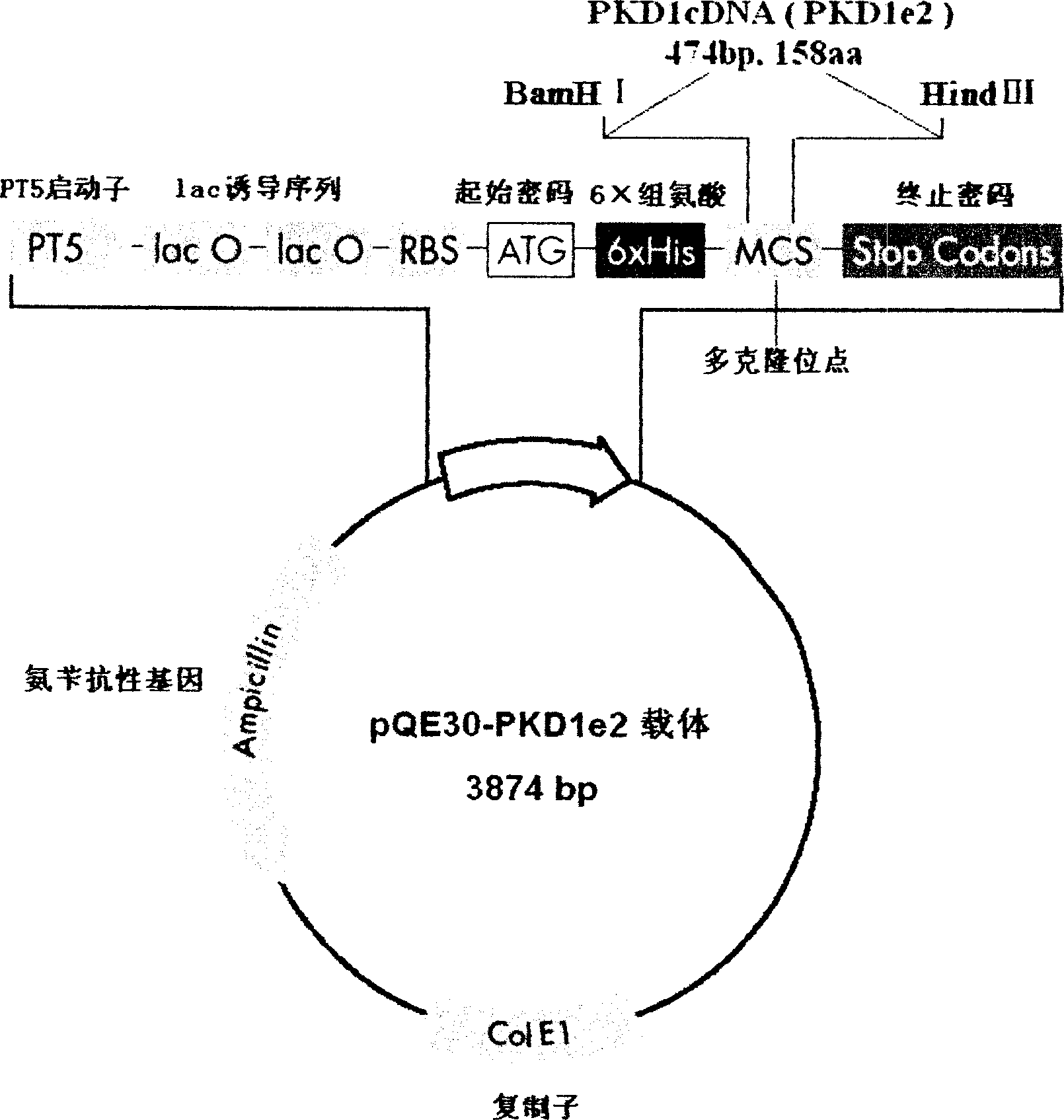
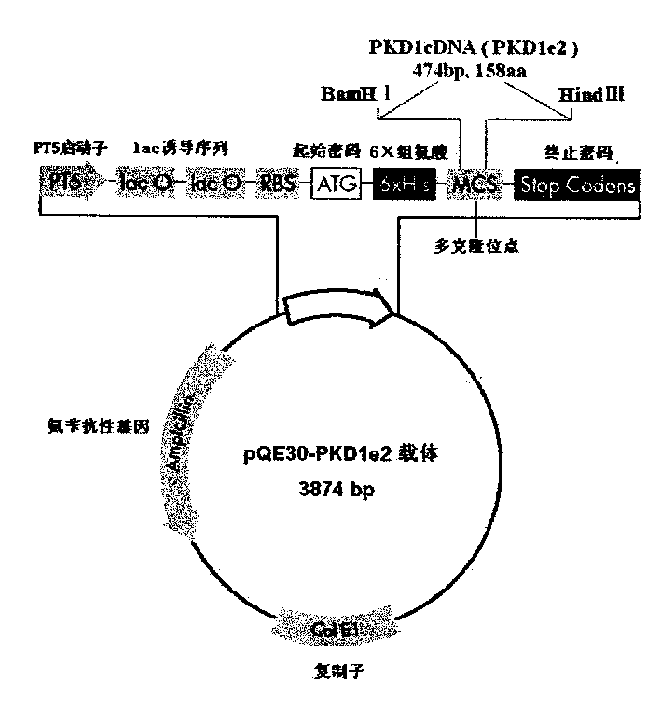
[0025] The preparation of human PC-1 N-terminal fusion protein PC-1-e2 is now described in detail.
[0026] 1. Materials
[0027] 1. Plasmids and strains: fusion protein expression vector pQE30 (the 5' end of its reading frame contains a nucleotide sequence encoding 6 consecutive histidines, and the plasmid contains an ampicillin resistance marker gene) and expression host bacterium M15 (the strain A repressor plasmid pREP4, which contains a kanamycin resistance marker gene) was purchased from Qiagen, Germany.
[0028] 2. Main reagents and instruments The total RNA extraction kit and gel recovery kit are products of Huashun Company, and the One-Step RT-PCR kit and Ni-NTA purification reagent (Ni-NTA Agarose) were purchased from Qiagen, Germany. Various restriction enzymes, T 4 DNA ligase is a product of Takara Company. Ampicillin (Amp) and Kanamycin (Kan) were purchased from Shanghai Huamei Bioengineering Company. Fetal bovine serum, RPMI 1640 and DMEM cell culture medium ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com