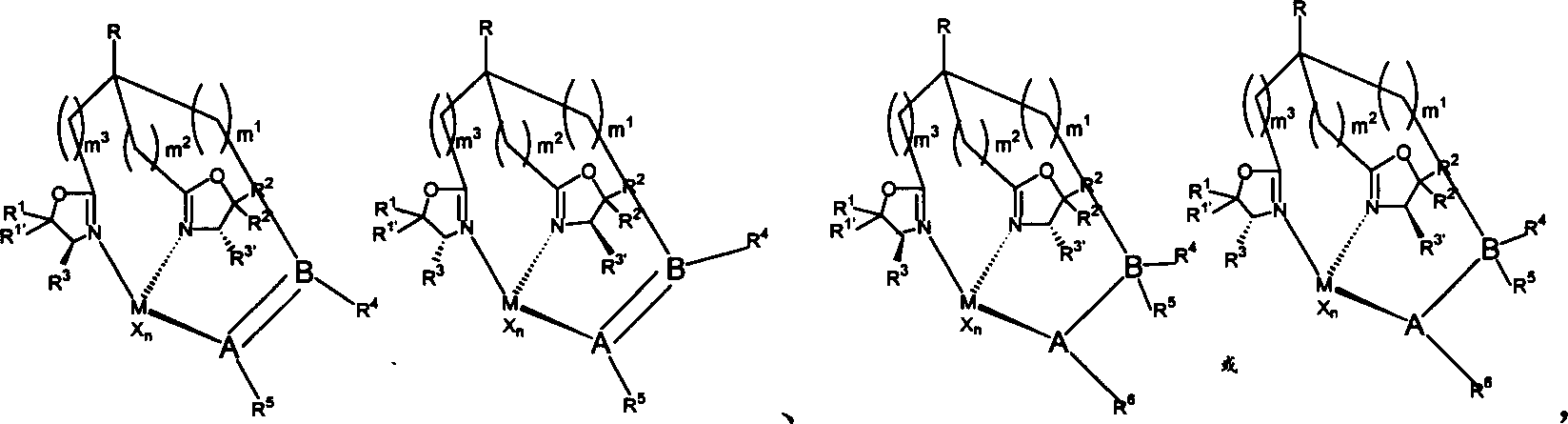
Multidentate oxazoline ligand having chirality and its compounding product with main group metal or transition metal, synthesis method and its use
A multidentate oxazoline and transition metal technology, which is applied in the field of catalytic asymmetric synthesis, can solve the problems of tediousness, failure to obtain good results, troublesome operation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Ligand L 1 Synthesis:
[0100]
[0101] Add 3.72g 1 (17mmol) and L-Valinol 7.04g (68mmol) to a 25ml Schlenk bottle, heat up to 80°C, the reaction system becomes a homogeneous transparent solution, stir, every 12 hours of heating, cool to 30°C, then vacuum methanol for about One hour, the cumulative reaction is 80 hours. Stop the reaction, dissolve with a small amount of dichloromethane, and perform direct flash chromatography with eluent (ethyl acetate / methanol=30 / 1) to obtain 4.52 g of product S1 as a white solid, with a yield of 61%. [α] D 20 =20.5° (c=1.09, HCl 3 ); 1 H NMR (300MHz, CDCl 3 ): δ0.93(m, 18H), 1.60(s, 3H), 1.65-1.90(m, 3H), 2.50(ABd, J=14.1Hz, 1H), 3.25(ABd, J=14.1Hz, 1H) , 3.32-3.42(m, 1H), 3.45-3.65(m, 3H), 3.67-3.90(m, 8h), 6.93(d, J=9.2Hz, 1H), 8.2(d, J=9.0Hz, 1H ), 8.8(d, J=8.1Hz, 1H); 13 C NMR (75MHz, CDCl 3 ): 174.33, 173.44, 173.30, 63.39, 62.90, 62.73, 60.424, 57.51, 57.23, 57.11, 53.752, 42.87, 29.43, 29.18, 29.10, 27.21, 27.14, 1...
Embodiment 2
[0104] Ligand L 2 Synthesis:
[0105]
[0106] Add 2.45g (11.2mmol) 1, 5.2g (45.0mmol) of L-Isoleucinol to a 25ml Schlenk bottle, heat up to 70°C, the reaction system becomes a homogeneous transparent solution, stir, every 12 hours of heating, cool to 30°C and vacuum pump Methanol is about one hour, and the cumulative reaction is 80 hours. Stop the reaction, after dissolving with a small amount of dichloromethane, directly flash chromatography, eluent (ethyl acetate / methanol=30 / 1), get the product S 2 It is 2.26 g of white solid, and the yield is 43.5%. 1 H NMR (300MHz, CDCl 3 ): δ0.94(m, 18H), 1.05-1.12(m, 3H), 1.38-1.58(m, 6H), 1.58(s, 3H), 1.90(br, 3H), 2.49(ABd, J=14.1 Hz, 1H), 3.22(ABd, J=14.1Hz, 1H), 3.3-4.0(m, 9H), 6.95(d, J=8.2Hz, 1H), 8.18(d, J=8.6Hz, 1H), 8.86 (d, J=9.2Hz, 1H). MS (m / z): 474 (M+1) + , (2), 357, (100), 313(67), 295(48), 69(40), 41(34), 55(26), 196(24), 339(24).
[0107] Add S to the 50ml three-necked bottle 2 750mg (1.6mmol), triphenylphos...
Embodiment 3
[0109] Ligand L 3 Synthesis:
[0110]
[0111] Add 1.33g (6.1mmol) 3.72g (27mmol) of 1,D-phenylglycinol to a 25ml Schlenk bottle, heat up to 90°C, the reaction system becomes a homogeneous transparent solution, stir, and cool to 30°C every 12 hours of heating After that, the methanol was vacuumed for about one hour, and the cumulative reaction was 60 hours. Stop the reaction, dissolve with a small amount of dichloromethane, and perform direct flash chromatography with an eluent (ethyl acetate / methanol=25 / 1) to obtain 1.06 g of product S3 as a white solid, with a yield of 32.6%. [α] D 20 =-102.3° (c=1.14, HCl 3 ); 1 H NMR (300MHz, CDCl 3 ): δ1.54(s, 3H); 1.63(br, s, 3H), 2.63(ABd, J=15.0Hz, 1H), 3.2(AB, J=15.0Hz, 1H), 3.6-4.1(m, 6H), 5.1-5.28(m, 3H), 7.28(m, 15H), 7.47(d, J=9.5Hz, 1H), 8.63(d, J=8.1Hz, 1H), 9.45(d, J=8.3 , 1H), 13 CNMR (75MHz, CDCl 3 ):173.6,173.1,172.7,138.6,138.4,128.73,128.66,127.7,127.6,126.73,126.71,126.43,66.43,65.69,65.51,56.05,55.60,53.38,4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com