Process for preparing chicken colibacillus pilus monoclonal antibody
A technology of monoclonal antibody and chicken Escherichia coli, which is applied in the direction of bacteria and fermentation, and can solve the problems of difficult standardization, large differences between batches, and low titer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0089] Embodiments of the present invention are described below in conjunction with accompanying drawings:
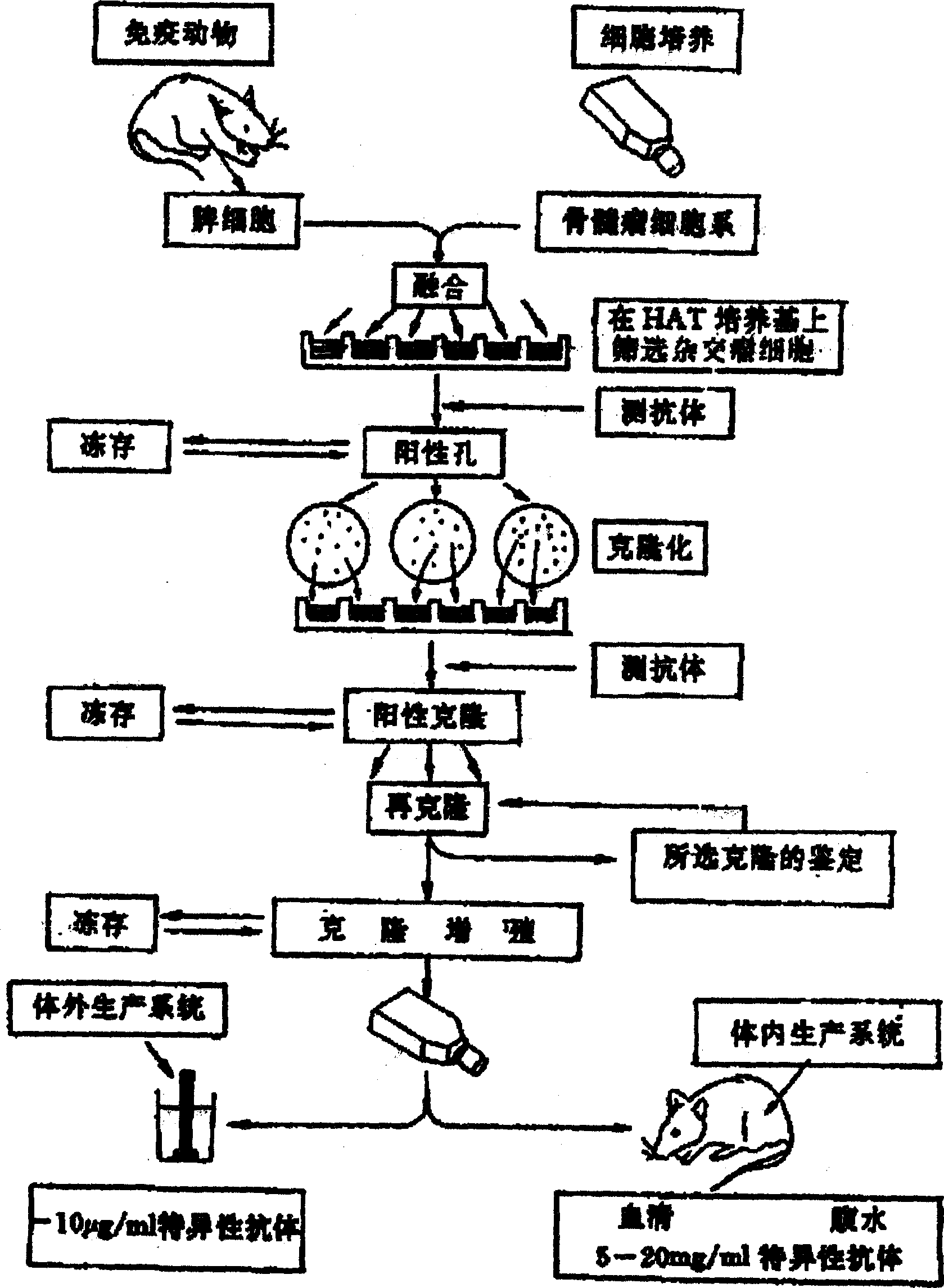
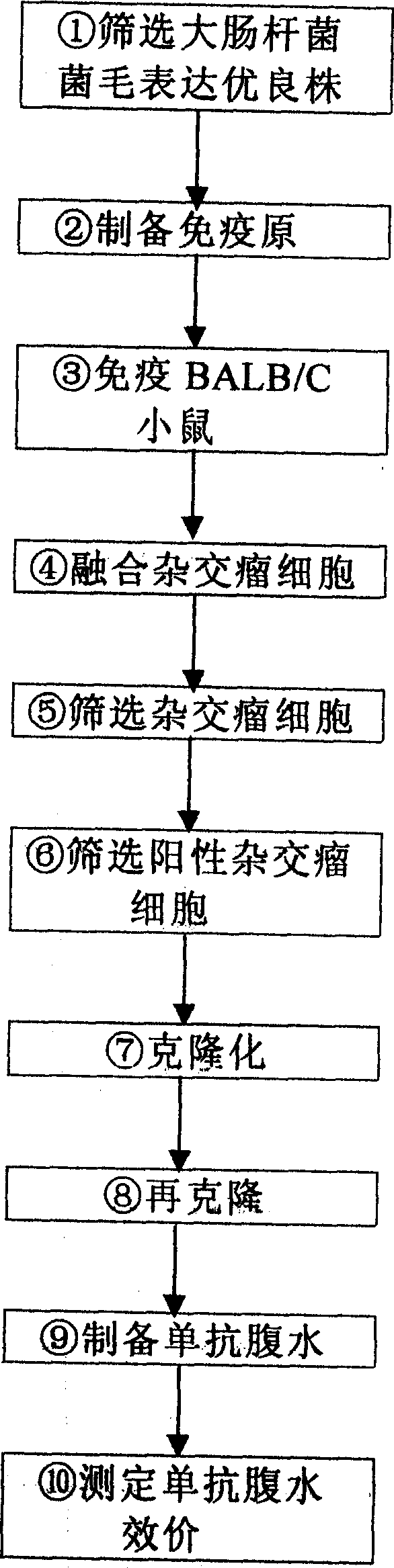
[0090] figure 1The preparation process of the monoclonal antibody of the embodiment of the present invention is described. It can be seen from the figure that the splenic lymphocytes of BALB / C mice immunized with predetermined antigens are fused with myeloma cells (SP2 / 0) which can grow unrestricted in vitro to form hybridoma cells with parental characteristics. The hybridoma cells can not only synthesize and secrete antibodies against predetermined antigens like splenic lymphocytes, but also live forever in vitro like myeloma cells. A hybridoma cell line from a single cell can be obtained by cloning, and the antibody secreted by it is directed against the same antigenic determinant and is molecularly homogeneous, the so-called monoclonal antibody (Monoclonal antibody), referred to as monoclonal antibody .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com