Process for preparation of dinapsoline
An independent, compound technology, applied in the field of dinaporine and some derivatives, can solve problems such as unreproducible yields and problems with cyclization steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
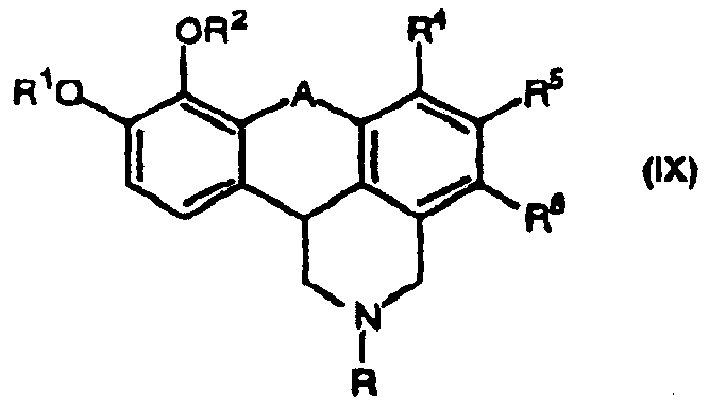
[0007] The present invention relates to a new method for preparing denatoprine and its derivatives for treating motor diseases and having the following structure, where R 1 and R 2 each independently is hydrogen or a hydroxyl protecting group; or R 1 and R 2 can be linked together to form -(CH 2 ) n -; n is 1-3; A is CH 2 、CHOR 1 or C=O; and R 4 , R 5 and R 6 each independently hydrogen, C 1-4 Alkyl, C 1-4 Alkoxy, hydroxy or halogen. This new and improved method is exemplified in Reaction Scheme 4.
[0008] The present invention also provides certain denapoline derivatives of formula IX for use in the treatment of motor disorders.
[0009] As used herein and in the claims (unless the context dictates otherwise), the term "C 1-4 "Alkyl" refers to straight chain or branched chain alkyl such as methyl, ethyl, propyl, isopropyl, butyl, isobutyl and tert-butyl. As used herein and in the claims, the term "C 1-4 "Alkoxy" means a straight or branched chain alkoxy group...
Embodiment 15
[0042] Embodiment 15-bromoisoquinoline
[0043] The experimental setup consisted of a 500ml three-neck flask equipped with a condenser, a dropping funnel, and a stirrer terminated in a rigid crescent-shaped Teflon polytetrafluoroethylene slurry. AlCl was added to a flask containing isoquinoline (57.6 g, 447 mmol) 3 (123g, 920mmol). The mixture was heated to 75-85°C. Bromine (48.0 g, 300 ml) was added dropwise over a period of 4 hours using a dropping funnel. The resulting mixture was stirred at 75°C for 1 hour. Pour the almost black mixture over crushed ice and stir well by hand. The cooled mixture was treated with aqueous sodium hydroxide (10N) to dissolve all aluminum salts as sodium aluminate and the oil layer was extracted with ether. use Na 2 SO 4 After drying and concentration, the ether extract was distilled at 0.3 mm. A white solid (16.3 g, 78 mmol) was obtained from the fraction at about 125°C (26% yield). The product was further purified...
Embodiment 25
[0050] Embodiment 25-isoquinoline formaldehyde (isoquinolinecarboxaldehyde)
[0051] At -78°C, add bromoisoquinoline (5.0 g, 24 mmol) in THF dropwise to n-butyllithium (19.3 ml 2.5 M n-hexane solution, 48 mmol) in ether (80 ml) and THF (80 ml) mixture solution (10ml) solution. The reaction mixture was heated at -78°C for 30 minutes. A solution of DMF (3.30 g, 45 mmol) in THF (10 ml) was cooled to -78 ℃ and quickly added to the lithium isoquinolate solution. The mixture was stirred at -78°C for 15 minutes. Ethanol (20ml) was added followed by NH 4 Cl solution. The suspension was warmed to room temperature. The organic layer combined with the ether extract was washed with Na 2 SO 4 dry. Pale yellow solid (2.4 g, 15 mmol, 64% yield) was obtained by chromatography (SiO 2 Type-H, 50% EtOAc / hexane) and recrystallized (ethanol): mp 114-116 °C;
[0052] 1 H NMR (DMSO-d 6 )δ10.40(s, 1H), 9.44(s, 1H), 8.85(d, 1H, J=6.0Hz),
[0053] 8.69(d, 1H, J=6.0H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com