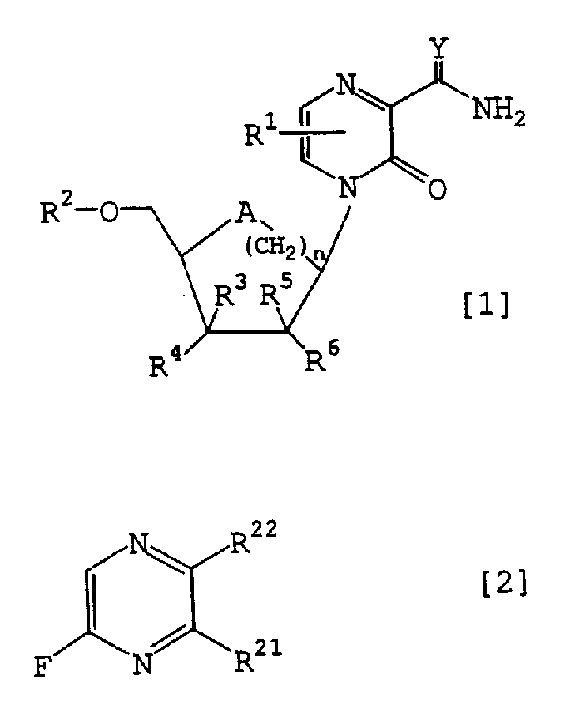
Novel pyrazine derivatives or salts thereof, containing the derives or the salts and intermediates for the preparation of both
A technology of pyrazine derivatives and compositions, applied in the field of new pyrazine derivatives or their salts, pharmaceutical compositions containing the derivatives or salts, and intermediates for the preparation of the two
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example I-2
[0417] In a nitrogen atmosphere, 11.4g of 6-bromo-3-methoxy-2-pyrazinecarboxylic acid methyl ester was dissolved in 227mL of toluene, and 10.3g of benzophenone imine, 0.42g of tris(dibenzylidene Acetone) dipalladium, 0.86 g (s)-(-)-2,2'-bis(diphenylphosphino)-1,1'-binaphthyl, and 6.20 g sodium tert-butoxide. The resulting mixture was stirred at 80 °C for one hour. After cooling the reaction mixture was filtered. The filtrate was purified by column chromatography (eluent: toluene:ethyl acetate=20:1). The obtained oily product was dissolved in 140 mL of tetrahydrofuran, 7 mL of 2 mol / L hydrochloric acid was added, and the resulting mixture was stirred at room temperature for 15 minutes. A mixture of 200 mL of chloroform and 50 mL of water was added to the reaction mixture, and then 1 mol / L sodium hydroxide was added to basify the mixture, and the organic layer was separated. The resulting organic layer was washed with a saturated aqueous solution of sodium chloride, dried ove...
reference example I-10
[0452] In 6.0 mL of N,N-dimethylformamide, 0.24 g of methyl 3-oxo-3,4-dihydro-2-pyrazinecarboxylate was dissolved. After adding 82 mg of 18-crown-6-ether and 62 mg of sodium hydride, the resulting mixture was heated at 80° C. for one hour. Then, 0.30 g (4aR, 7S, 8aR)-2-phenylhexahydropyrano[3,2-d][1,3]dioxan-7-yl 4-methylbenzenesulfonate was added dropwise 3.0 mL of N,N-dimethylformamide solution, and the resulting mixture was heated at 100°C for 4 hours. The reaction mixture was allowed to cool, diluted with 50 mL of ethyl acetate and 25 mL of water, and the layers were separated. Further, the aqueous layer was extracted with three 25 mL portions of ethyl acetate. All the obtained organic layers were combined, washed successively with a saturated aqueous solution of sodium bicarbonate and a saturated aqueous solution of sodium chloride, dried over anhydrous magnesium sulfate, and the solvent was removed under reduced pressure. The resulting residue was purified by column c...
reference example I-11
[0456]In 5.7 mL of N,N-dimethylformamide, 0.38 g of methyl 3-oxo-3,4-dihydro-2-pyrazinecarboxylate was dissolved. After adding 0.10 g of sodium hydride, the resulting mixture was heated at 80° C. for 30 minutes. Then, 0.19 g (4aR, 7R, 8S, 8aR)-8-hydroxy-2-phenylhexahydropyrano[3,2-d][1,3]dioxan-7-yl 4-methanol was added The phenylsulfonate was heated at 100°C for an additional 4.5 hours. The reaction mixture was allowed to cool, diluted with 30 mL of ethyl acetate and 20 mL of water, and the resulting mixture was separated. Further, the aqueous layer was extracted with 30 mL of ethyl acetate. All the obtained organic layers were combined, washed successively with a saturated aqueous solution of sodium bicarbonate and a saturated aqueous solution of sodium chloride, dried over anhydrous magnesium sulfate, and the solvent was removed under reduced pressure. The resulting residue was purified by column chromatography (eluent: toluene:ethyl acetate=2:1), isopropyl ether and die...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com