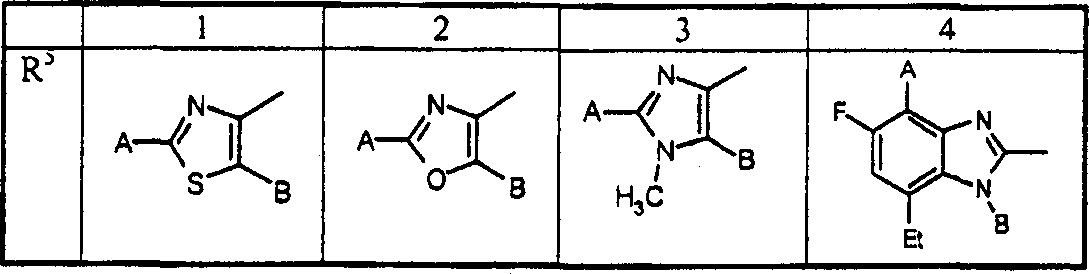
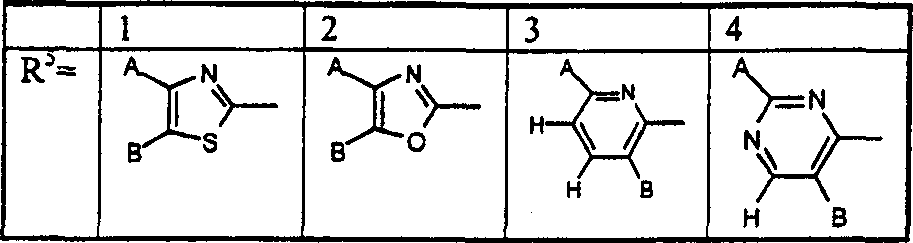
A combination of FBPase Inhibitors and insulin sensitizers for the treatment of diabetes
A technology of insulin and sensitizers, applied in the field of therapeutic compositions, can solve problems such as weak activity, inability to inhibit glucose production, and poor cell penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0770] Compounds of formula VI are prepared according to modified literature methods and other methods well known to those skilled in the art. These compounds are generally synthesized by the method of Srivastava, J. Med. Chem. (1976). Wood et al., J. Med. Chem. 28: 1198-1203 (1985); Sagi et al., J. Med. Chem. 35: 4549-4556 (1992); Paul, Jr. J. Med. Chem. 28: 1704-1716 (1985); Cohen et al., J. Am. Chem. Soc. 95:4619-4624 (1973). Other methods are described.
[0771] Compounds of formula II-IV were prepared according to the methods described in PCT publications WO 98 / 39344, WO 98 / 39343, and WO 98 / 39342. Part 1 Synthetic formula I compound
[0772] Synthesis of compounds within the scope of this invention generally includes some or all of the following general steps: (1) preparation of phosphonate prodrugs; (2) deprotection of phosphonate; (3) modification of heterocycle; Ring coupling with phosphonate moieties; (5) construction of heterocycles; (6) ring closure to construct ...
Embodiment Embodiment 1 preparation 5- 2 -2-(1)A. -78℃,2- 2 (1mmol)THF( 4 )nBuLi(1mmol)。1, 2 (1.2mmol),40。,。B. 90℃80%4。,1,。 or preparation 。C. -78℃,(1mmol)TMEDA(N,N,N’,N’- 4 2 )(1mmol) and nBuLi(2mmol)0.5。 2 (1.2mmol),1。,2- 2 ,。D. -78℃,2- 2 (1mmol)THFLDA(1.12mmol,N,N- 2 )20。(1.5mmol),1。,1,。2- preparation 。E. 2-(1mmol) and N,N’- 2 2 (1mmol),-。2,,,-2-(N,N’- 2 ),。bp 59-61℃(3mmHg)。F.-40℃--48℃,-2-(N, N’- 2 )(1mmol) and TMEDA(1mmol)THFnBuLi(1.3mmol)。0℃1.5, to -55℃, 2 (1.1mmol)THF。25℃12,,,5- 2 -2-(N,N’- 2 ),。G. 5- 2 -2-(N,N’- 2 )(1mmol) to pH=1。,1,。 Embodiment 2
[0822]Examples Example 1 Preparation of 5-diethylphosphono-2-furfuraldehyde (1) Step A. At -78°C, a solution of 2-furfuraldehyde diethyl acetal (1 mmol) in THF (tetrahydrofuran) was washed with nBuLi (1 mmol) treatment. After 1 hour, diethyl chlorophosphate (1.2 mmol) was added and the reaction was stirred for 40 minutes. After extraction and evaporation, a brown oil was obtained. Step B. The resulting brown oil was treated with 80% acetic acid at 90°C for 4 hours. After extraction and chromatographic purification, compound 1 was obtained as a clear yellow oil. Alternatively the aldehyde can be prepared from furan as described below. Step C. A solution of furan (1 mmol) in ether was treated with TMEDA (N,N,N',N'-tetramethylethylenediamine) (1 mmol) and nBuLi (2 mmol) at -78°C for 0.5 h. Diethyl chlorophosphate (1.2 mmol) was added to the reaction mixture and stirred for another 1 hour. Extraction and distillation afforded diethyl 2-furanphosphonate as a clear oil. Step D...
Embodiment 3
[0828] The following compounds were prepared according to this method: (2.4) 5-diethylphosphono-2-(2-ethoxycarbonylacetyl) furan (2.5) 5-diethylphosphono-2-(2- Methylthioacetyl)furan (2.6) 6-diethylphosphono-2-acetylpyridine Example 3. Preparation of 4-[(2-(5-phosphono)furyl]thiazole, 4-[2- (6-phosphono)pyridyl]thiazole and 4-[2-(5-phosphono)furyl]selenazole Step A. A solution of compound 2.1 (1 mmol) in ethanol was treated with copper(II) bromide (2.2 mmol) was treated under reflux conditions for 3 hours. The cooled reaction mixture was filtered and the filtrate was evaporated to dryness. The resulting dark oil was purified by chromatography to obtain 5-diethylphosphono-2-[(2- Bromo-4-methyl-1-oxo)pentyl]furan as an orange oil. Step B. Adding 5-diethylphosphono-2-[(2-bromo-4-methyl-1-oxo A solution of pentyl]furan (1 mmol) and thiourea (2 mmol) in ethanol was heated to reflux for 2 hours. The cooled reaction mixture was evaporated to dryness and the resulting yellow foam was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com