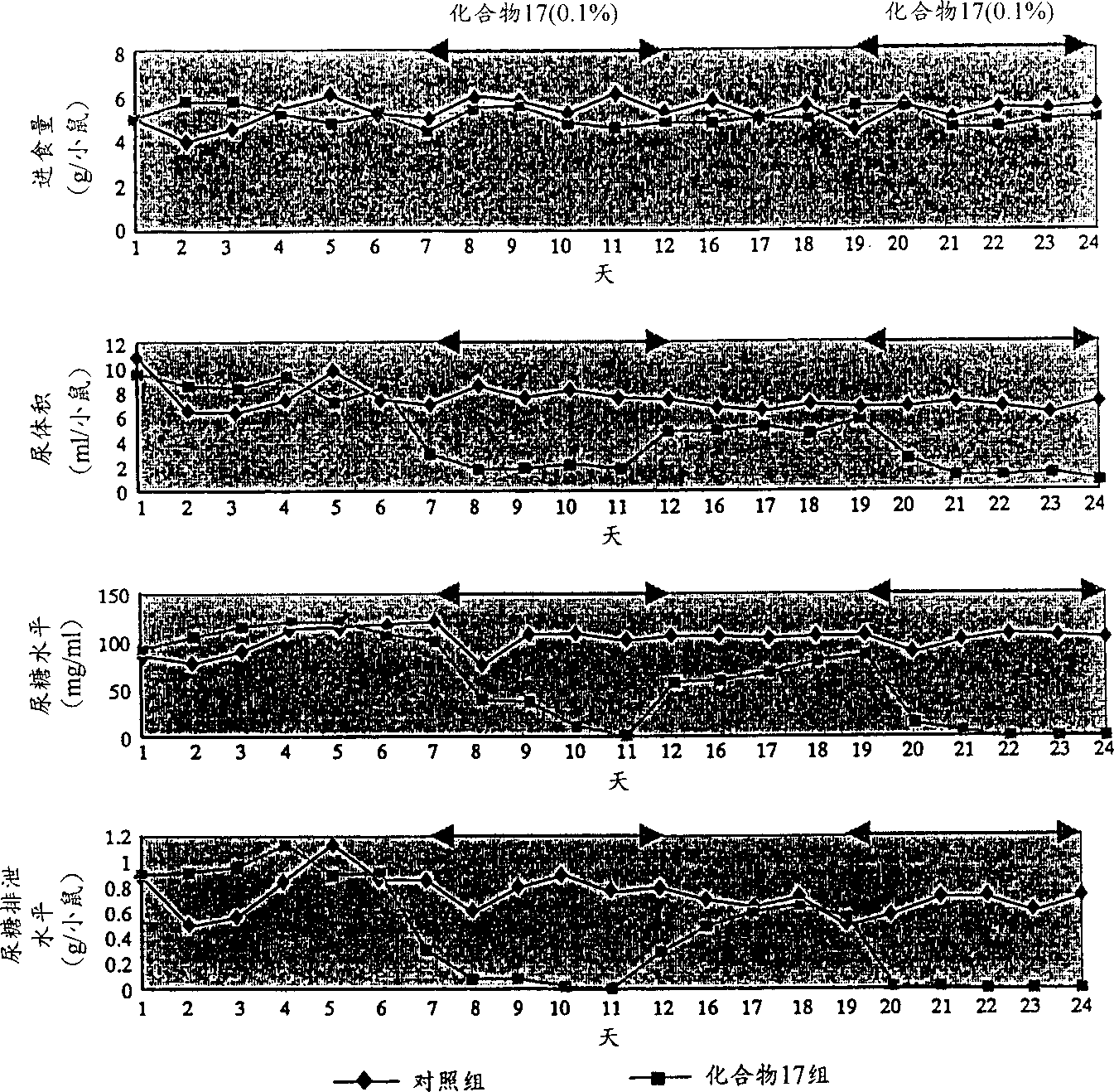
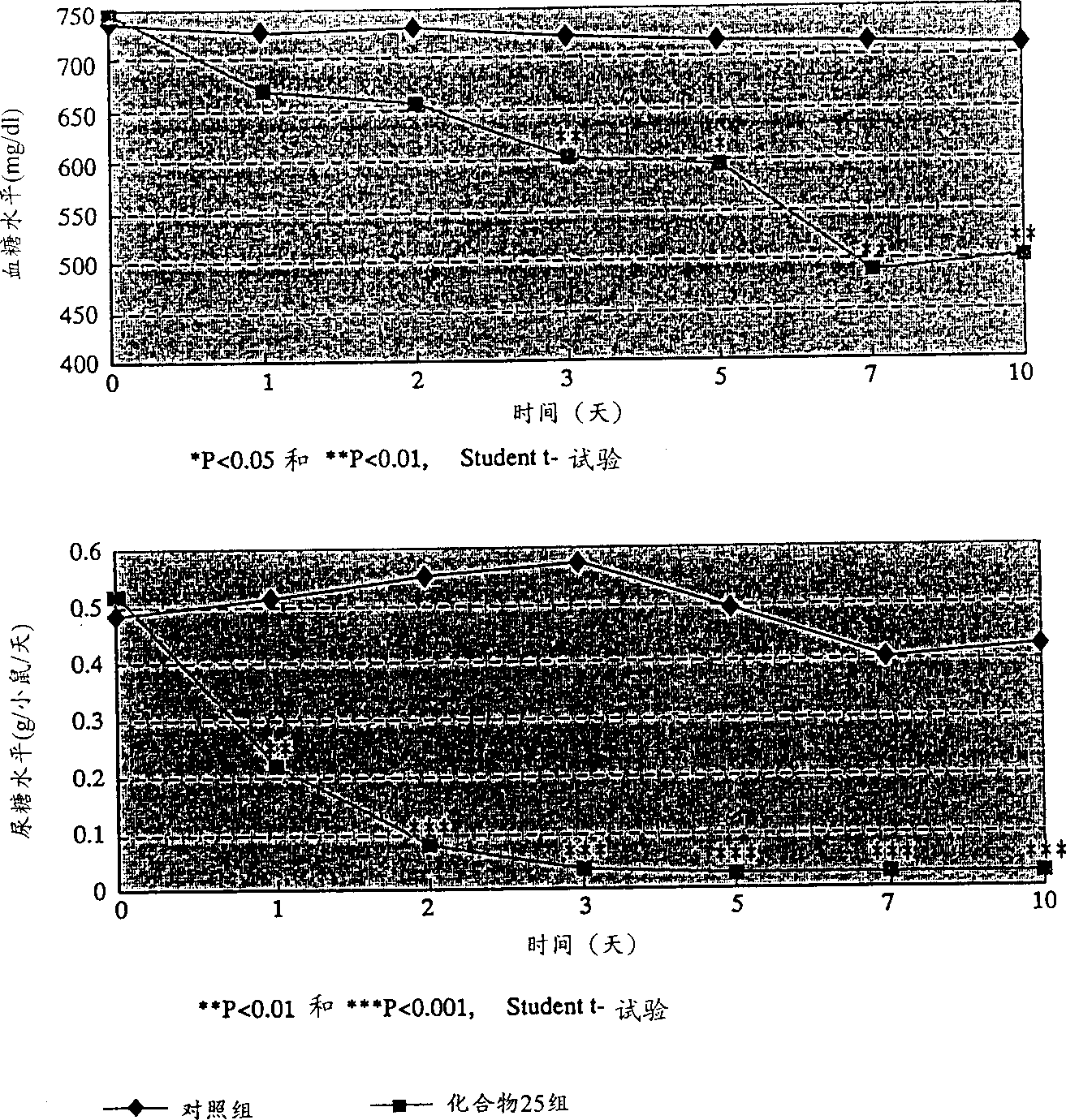
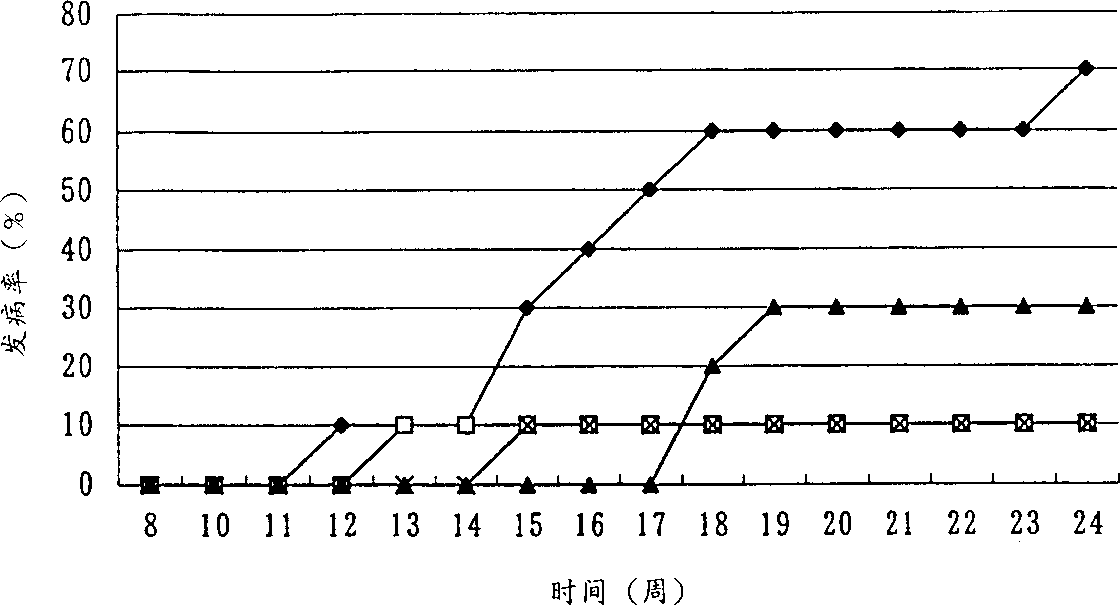
Novel ligands of nuclear receptors PPAR's
A technology of nuclear receptors and ligands, applied in medical preparations containing active ingredients, receptors/cell surface antigens/cell surface determinants, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0102] Dehydro ascofuranone
[0103] The synthesis of dehydro ascofuranone by removing two hydrogen atoms from the tetrahydrofuran ring of ascofuranone is as follows. Under water bath cooling, add AgNO to 0.16g (4.0mmol) sodium hydroxide in 0.7ml deionized water solution in batches 3 (0.34g, 2.00mmol) in deionized water (0.7ml). A solution of ascofuranone (0.2 g, 0.476 mmol) in dioxane (1.4 ml) was added to the resulting aqueous suspension of silver nitrate. The reaction mixture was stirred at room temperature for 3 hours, then diluted with water / dioxane mixed solvent (1:1), filtered through celite, the filtrate was acidified with 6N HCl, and then extracted with dichloromethane. The dichloromethane layer was washed with water and saturated brine, and then subjected to MgSO 4 Dry and concentrate under reduced pressure. The residue was purified by silica gel II column chromatography and eluted with dichloromethane / acetone (60:1) to obtain 0.047 g of dehydro ascofuranone (yield 24%)....
preparation example 2
[0107] A. 4-O-Ethoxycarbonylmethyl-2-O-methyl ascochlorin (1): compound 27
[0108]Sodium hydroxide (60%, 23.1 mg, 0.578 mmol) was washed with pentane and then suspended in DMF (1 ml). To this suspension was added dropwise a solution of 2-O-methyl ascochlorin (0.220g, 0.525mmol) in DMF (2ml), the mixed solution was stirred at room temperature for 30 minutes, and then mixed with ethyl bromoacetate ( 0.0641ml, 0.578mmol) was stirred at 50°C for 4 hours. The reaction solution was dissolved in a saturated aqueous ammonium chloride solution, extracted with ether, and the organic layer was successively washed with water and saturated brine, and then dried over anhydrous magnesium sulfate. After filtering off the desiccant, the filtrate was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography (15g SiO 2 , Hexane / ethyl acetate), 0.204 g (77%) (1) (colorless gum) was obtained.
[0109] IR (thin film): 1765, 1713, 1694. NMR (CDCl 3 , 500MHz):...
preparation example 3
[0115] 4-O-(4-carboxybutyryl) ascochlorin (4): compound 29
[0116] To ascochlorin (0.150 g, 0.358 mmol) in anhydrous pyridine (1.5 ml) was added 4-dimethylaminopyridine (4.4 mg, 0.036 mmol) and glutaraldehyde (0.049 g, 0.430 mmol). The reaction solution was stirred at 50°C overnight, then adjusted to pH 2 with 1N hydrochloric acid, and extracted with ethyl acetate. The organic layer was washed with saturated brine, and then dried over anhydrous magnesium sulfate. After filtering the desiccant, the filtrate was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography (13g SiO 2 , Hexane / ethyl acetate) to obtain 0.081 g (42%) (4) as colorless crystals.
[0117] mp 120-123°C.IR (KBr disk): ~ 3000, 1771, 1711, 1649. NMR (CDCl 3 , 500MHz): 0.70 (3H, s), 0.81 (3H, d, J = 7.0 Hz), 0.83 (3H, d, J = 7.0 Hz), 1.59-1.66 (1H, m), 1.88 (3H, s) , 1.89-1.96 (2H, m), 2.12 (2H, qui, J=7.2Hz), 2.34-2.45 (3H, m), 2.55 (2H, t, J=7.2Hz), 2.65 (3H, s), 2.76...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com