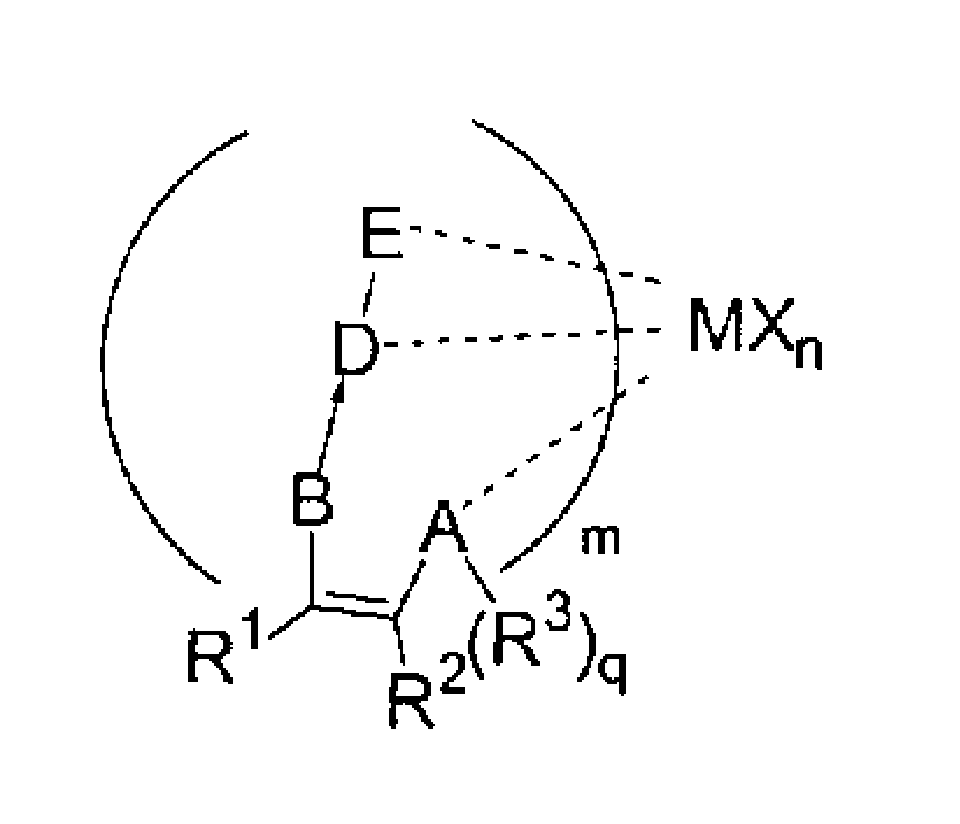
Catalyst for polymerization and copolymerization of olefine and its synthesis and use
A technology of olefin polymerization and copolymerization, which is applied in the fields of olefin polymerization and copolymerization catalysts, synthesis and its application, and can solve problems such as compound unused olefin polymerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0221] Example 1 Synthesis of Ligand L1
[0222] In a 100ml reaction flask, add 8.2g (34.4mmol) 3,5-di-tert-butyl salicylaldehyde, 9.6g (34.6mmol) (o-aminophenyl) diphenylphosphine, 50ml absolute ethanol, molecular sieves And a catalytic amount of glacial acetic acid, heated to reflux for 24h, stopped the reaction, removed molecular sieves, concentrated, allowed to cool to room temperature to obtain the crude product, recrystallized with ethanol / ether to obtain light yellow crystal L1, 11.8g (69.4%) .
[0223] Elemental analysis: Measured (calculated value): C: 80.39 (80.29) H: 7.59 (7.35) N: 2.77 (2.84)
[0224] 1 HNMR (300MHz CDCl 3 ): δ8.4 (s CH=N); 7.4-6.8 (m, Aryl H) 1.4, 1.3 (s, s t-Bu-H)
[0225] δ( 31 P)-5.52(s)
Embodiment 2
[0226] Example 2 Synthesis of Ligand L2
[0227] In the reaction bottle of 50ml, add 750mg (3.2mmol) 3, the glacial acetic acid of 5-di-tert-butyl salicylaldehyde, 440mg (3.2mmol) o-dimethylaminoaniline, 25ml dehydrated alcohol, molecular sieve and catalytic amount, After heating to reflux for 20 h, the reaction was stopped, molecular sieves were removed, concentrated, and allowed to cool to room temperature to obtain a crude product, which was recrystallized from ethanol to obtain 352 mg (31%) of light yellow crystal L2.
[0228] Elemental analysis: measured (calculated value): C78.20 (78.37) H8.86 (9.l7) N7.86 (7.86) 1 HNMR (300MHz CDCl 3 ): δ (ppm) 13.9 (s, O-H) 8.6-8.7 (s CH = N) 7.6-6.9 (m, Aryl6H) 2.9 (s, CH 3 6H) 1.5, 1.3 (s, st-Bu-H, 9H, 9H)
[0229] MS352(M+100.00)
[0230] IR (KBr tablet) υ C=N (cm -1 )1614
Embodiment 3
[0231] Example 3 Synthesis of Ligand L3
[0232] In the reaction flask, add 1.17g (5.25mmol) 3,5-di-tert-butyl salicylaldehyde, 0.64g (5.25mmol) (N-methyl o-phenylenediamine), 15ml absolute ethanol, molecular sieves and catalytic amount After heating to reflux for 2 hours, stop the reaction, remove the molecular sieve, and cool to obtain the crude product, which is recrystallized from ethanol to obtain light yellow crystal L31.67g (98%).
[0233] Elemental analysis: measured (calculated value): C77.85 (78.06) H.86 (8.98) N8.30 (8.28): 1 HNMR (300MHz CDCl 3 ): δ (ppm) 13.4-13.2 (s, O-H) 8.3 (s CH = N); 7.6-6.6 (m, 6HAryl-H) 3.1 (s, N-CH 3 ) 1.5, 1.4 (s, s 9H, 9H t-Bu-H).
[0234] MS(M+)338
[0235] IR(KBr)υ C=N (cm -1 )1613.3
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| crystallinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com