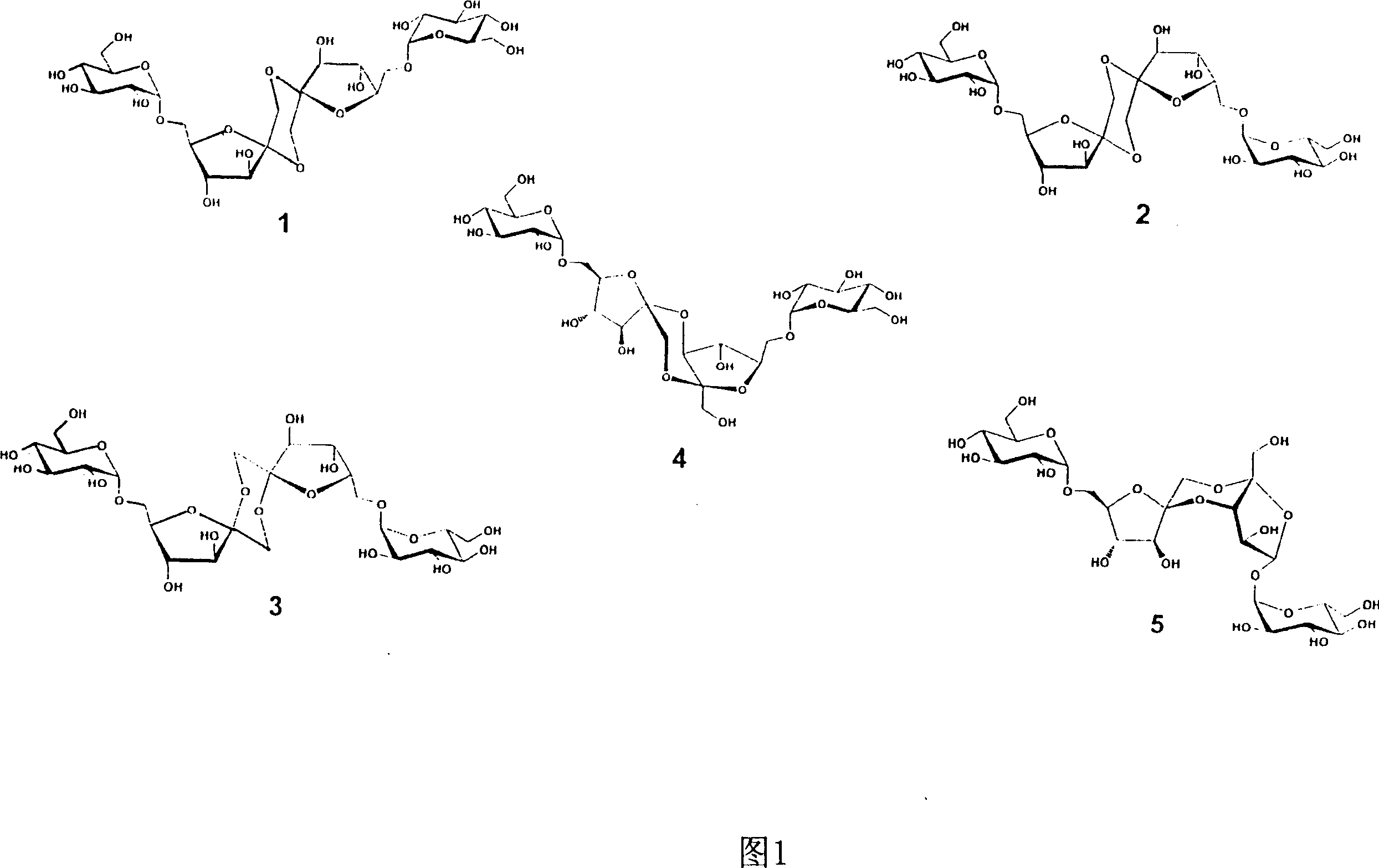
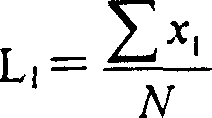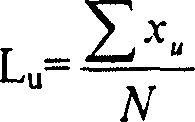Condensed palatinose and method for producing the same
A Palatinose, condensation technology, applied in the direction of confectionery, oligosaccharide, confectionery industry, etc., can solve the problem of limited use, etc., and achieve the effects of low enzymatic degradation, reduced siltation, and high availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1: Production of condensed palatinose according to prior art (comparative example)
[0089] Add 90g of deionized water in a steel container, dissolve 300g of crystalline palatinose in it, and stir at a temperature of 105°C, then add citric acid (equivalent to 0.02wt of palatinose used) %), and concentrated under vacuum until the final temperature reached 135°C. After reaching 135°C, the temperature was maintained for 30 minutes, followed by cooling and dissolving the reaction product with deionized water.
[0090] The composition of the reaction product, ie the DP range, can be determined by means of gel permeation chromatography, using Raftilose(R) St sweetener as reference substance. Here, range DP2 largely corresponds to uncondensed palatinose (isomaltulose).
[0091] Element
share
(weight percent)
Range DP1 (hydrolysis product)
2
Range DP2 (Uncondensed Palatinose)
48
...
Embodiment 2
[0094] Example 2: Production of condensed palatinose from the melt (according to the invention)
[0095] First, 800 g of deionized water containing 10 g of anhydrous citric acid was heated to 75° C. in a coking pan with a stirrer and a maximum working volume of about 20 L. Then, while stirring, add 10kg palatinose in small portions. After the addition was complete, the coking pan was heated to 145° C. at maximum heating power (4.4 kW) and maximum stirring speed, and reacted at 145° C. for 45 minutes. Subsequently, the resulting melt was quenched with 4 kg of deionized water, and the resulting syrup was discharged. Condensed palatinose is extracted from this syrup by known methods.
[0096] The fraction of the DP range was determined analytically by means of gel permeation chromatography with Raftilose(R) L40 and Raftiline(R) St as reference substances.
[0097] ingredients
weight percent
Before separating GMF
After separation of GMF
...
Embodiment 3
[0102] Example 3: Chromatographic concentration and impurity separation of palatinose condensates with a cation exchanger loaded with calcium ions
[0103] In order to separate the uncondensed palatinose contained in the reaction product obtained in Example 2, to concentrate the palatinose condensate, and / or to separate out impurities. According to the method described in Example 2, with a Ca 2+ A strongly acidic cation exchanger in the form of (eg Amberlite XE 594) for chromatographic separation.
[0104] Chromatographic separation:
[0105] Separation equipment: length 10m, diameter 25cm
[0106] Temperature: 55°C
[0107] Flow rate: 100L / h
[0108] Elution medium: distilled water
[0109] Charge solution: 34.4 kg containing 50 weight percent dry matter of the reaction product (equivalent to 17.4 kg dry matter)
[0110] result:
[0111] share in dry matter
[0112] Depending on the type and type of fractionation in the chromatographic separation step, the im...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com