Indezole bioisostere replacement of catechol in therafeutically active compounds
A biological isosteric and compound technology, applied in the fields of organic active ingredients, drug combinations, organic chemistry, etc., can solve the problems that the types of treatments do not conform to direct prediction and lack of structural similarity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
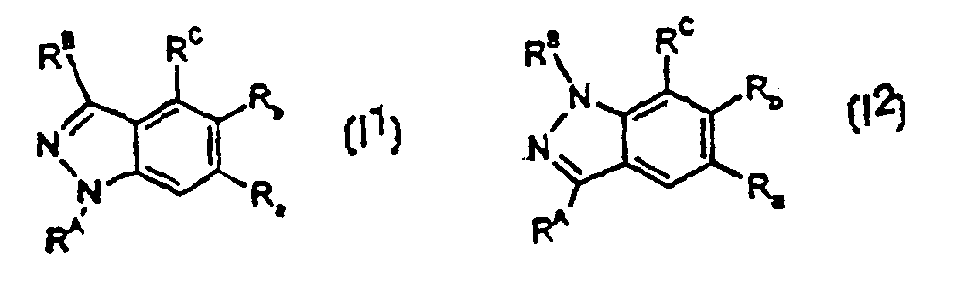
Image
Examples
preparation example Construction
[0733] The preparation of the compound of formula (I-ii) is further described in Example 23 below. The compound can also be prepared according to the synthetic methods described in the above scheme 2 and scheme 3, using the compound (represented by formula (9.53)) prepared by the method described in the following example 20 as the starting material:
[0734] Preferred compounds of the above formula (9.51) can be prepared following the synthetic methods described in Scheme 1, Scheme 2, and Scheme 3 above and in more detail in the Examples below. Additional preferred methods for the preparation of said compounds are also available and are shown in the following Synthetic Scheme 5, which is a more general representation of the methods for the preparation of the above-mentioned preferred compounds of the present invention.
[0735] Process 5
[0736] As shown above, the starting material of formula XXVIII is reacted with hydrazine of formula ...
Embodiment 1
[0793] A. 5-Benzyloxy-4-methoxy-2-{1[4-(morpholinecarbonyl)-1,4-diazepan-1-yl]ethyleneamino}benzonitrile
[0794] Phosphorus oxychloride (0.81ml, 0.0086mol) was added to 1-acetyl-4-(4-morpholinecarbonyl)-1,4-diazepane (4.02g, 0.0157mol) in dichloromethane (25ml) solution and the mixture was stirred at room temperature for 30 minutes. Then a solution of 2-amino-5-benzyloxy-4-methoxybenzonitrile (2 g, 0.0078 mol) in dichloromethane (25 ml) was added thereto, and the reaction was stirred at 40°C for 18 hours. After cooling, the reaction mixture was carefully poured into ice / water (100ml) and extracted with dichloromethane (2 x 100ml). The combined organic layers were washed with MgSO 4 Dry, filter, and distill under reduced pressure to obtain a brown oil. The crude product was purified on a silica gel column eluting with a gradient of methanol:dichloromethane (2:98 to 10:90 v / v) to afford the title compound. R f 0.67 (0.880 ammonia:methanol:dichloromethane 1:7:92, v / v). MS...
Embodiment 2
[0799] Contraction response of rabbit aorta
[0800] Rabbit aortic tissue was cut into rings and suspended under a resting tension of 1.5 g in an organ bath of Krebs Ringer bicarbonate (mM) of the following composition: NaCl (119), KCl (4.7), CaCl 2 (2.5), KH 2 PO 4 (1.2), MgSO 4 (1.2), NaHCO 3 (25), glucose (11), and use 95% / O 2 / 5%CO 2 saturation. The solution also contained 1 mM propanol, 0.5 mM idazoxan, 10 mM cocaine and 10 mM corticosterone. Tissues were exposed to two sensitizing doses of methoxyamine (100 mM) and washed over 1 hour. Control curves in all tissues were obtained from isotonic contraction in response to increased accumulation of methoxyamine. Further curves were obtained in the presence and absence of antagonist (1 hour incubation). Antagonist affinity estimates (pK b ), pK b =-log[A] / (DR-1) where the dose ratio (DR), relative to the corresponding control group, can be generated by the concentration of antagonist [A] alone which is assumed to be...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com