Method for purifying composite interferon
A technology of compound interferon and purification method, which is applied in the field of one-step purification of recombinant compound interferon with cation exchange medium, can solve the problems of low purity, uneven product structure and the like, achieves simple steps, saves process and operator, and is easy to use. The effect of promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment l
[0022] Embodiment 1, the production of composite interferon
[0023] The unpurified compound interferon used in the present invention is obtained by artificially constructing the compound interfering gene by conventional methods and fermenting the host cell containing the DNA sequence expression vector. The specific fermentation process is as follows:
[0024] Escherichia coli BL21 was selected as the host bacteria, and the strains were stored at -80°C. Pick compound interferon engineering bacteria (provided by researcher Tang Hong, Institute of Microbiology, Chinese Academy of Sciences) to streak on ammonia-resistant LB plates, and culture them in a greenhouse at 37°C for 12-24 hours. Pick a single colony, inoculate in 3 ml of liquid LB medium (ammonia, 1-50 mg / ml), culture on a shaker at 37°C at a speed of 240 rpm for 12-24 hours, measure OD 600 When it is 1.0-2.0, it is transferred to the seed solution twice, cultivated under the same conditions, and the OD 600 When it re...
Embodiment 2
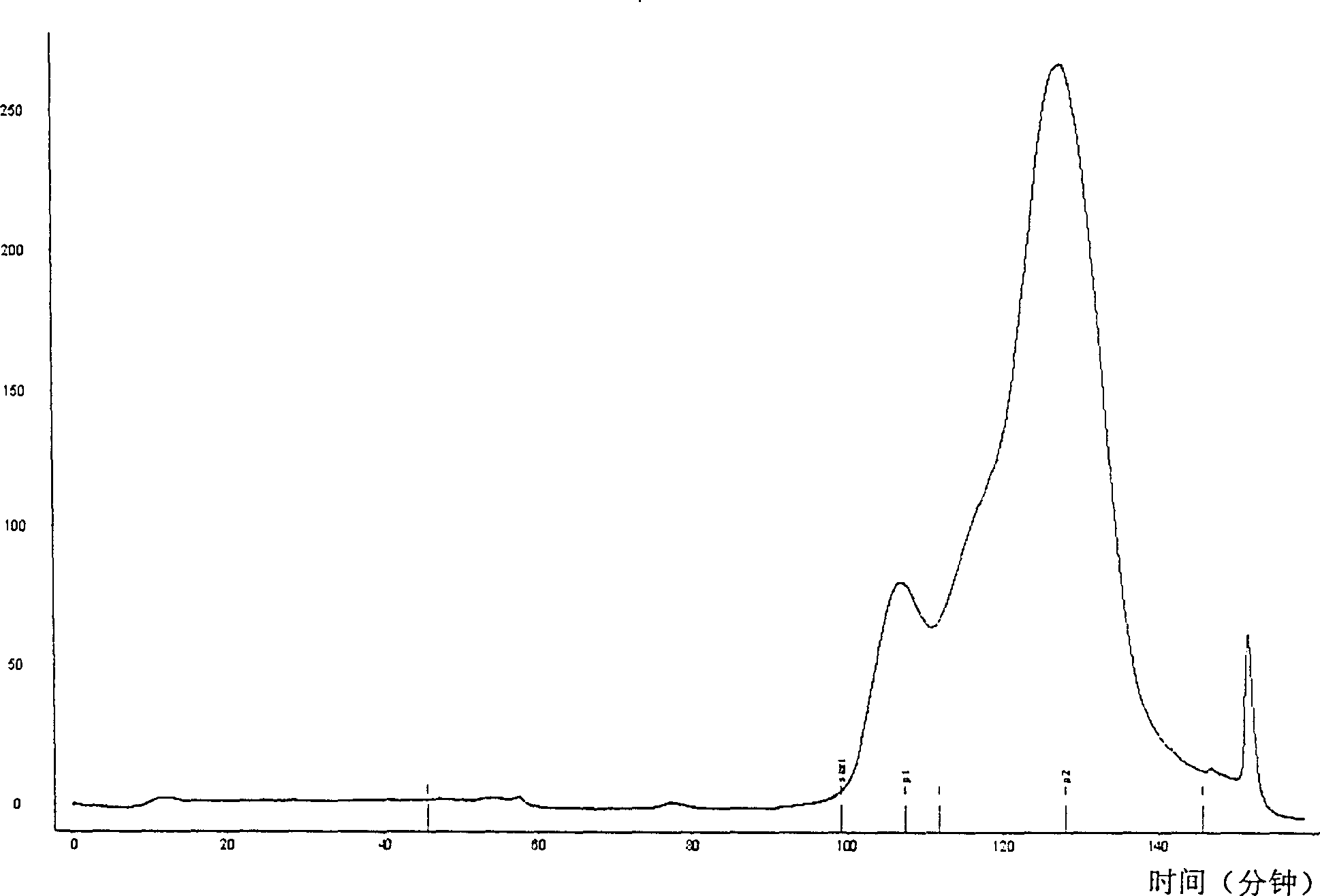
[0029] Embodiment 2, the purification of recombinant composite interferon
[0030] The compound interferon with a purity of 65% obtained by fermenting and collecting inclusion body denaturation by conventional methods in Example 1 was added buffer D (100mM Tris+0.5M arginine+0.2mM EDTA (ethylenediamine disodium tetraacetate), adjust the pH to 8.3 with HCl), the volume ratio of the added buffer solution D to the composite interferon is 40:1, and then the mixed solution is allowed to stand overnight.
[0031] Then put this mixed solution into a dialysis bag with a molecular weight of 8000, and dialyze in buffer E (25mM Tris-HCl, pH8.3) for 12 hours to perform renaturation of the composite interferon protein. The volume ratio is 5:1; the white aggregates are removed by centrifugation or filtration, and the supernatant is separated, which is the solution of refolded compound interferon. The concentration of refolded protein is 0.3mg / ml. The protein purity is 90%, and the protein ...
Embodiment 3
[0034] Embodiment 3, the purification of recombinant composite interferon
[0035] Composite interferon with a purity of 65% obtained by fermenting and collecting the inclusion bodies denatured by conventional methods in Example 1, adding buffer D (100mM Tris+0.5M arginine+0.2mM EDTA, adjusted with HCl) while stirring pH to 8.3), the volume ratio of the added buffer D and composite interferon is 100:1, and then the mixed solution is allowed to stand overnight;
[0036] Then put this mixed solution into a dialysis bag with a molecular weight of 50,000, and dialyze in buffer E (25mM Tris-HCl, pH8.3) for 24 hours to perform renaturation of the complex interferon protein. The volume ratio is 20:1; centrifuge or filter to remove white aggregates, separate the supernatant, which is the solution of refolded compound interferon, the concentration of refolded protein is 0.8mg / ml, and the compound interfered by SDS-PAGE The protein purity is 90%, and the protein refolding rate is 70%. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com