Derivate of sweetsop lactone as well as preparation method and usage
A technology of anemone lactone and derivatives, which is applied in the field of new anemone lactone derivatives, and can solve problems such as difficult chemical synthesis, many chiral centers, and complex structures
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
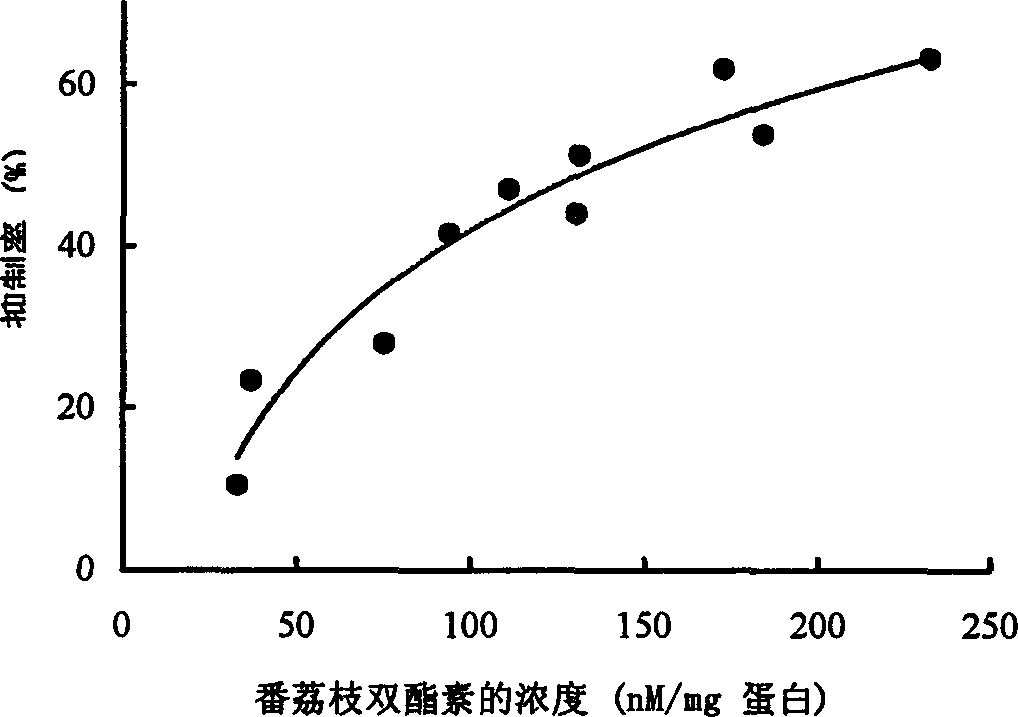
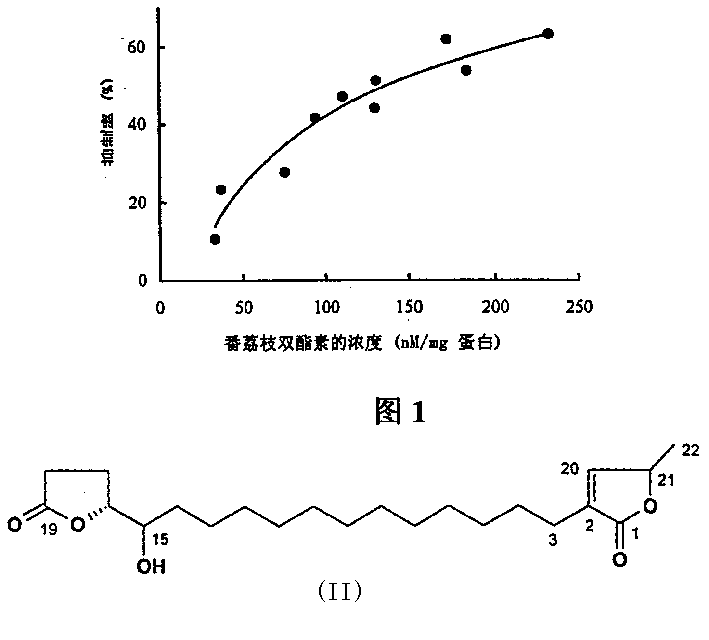
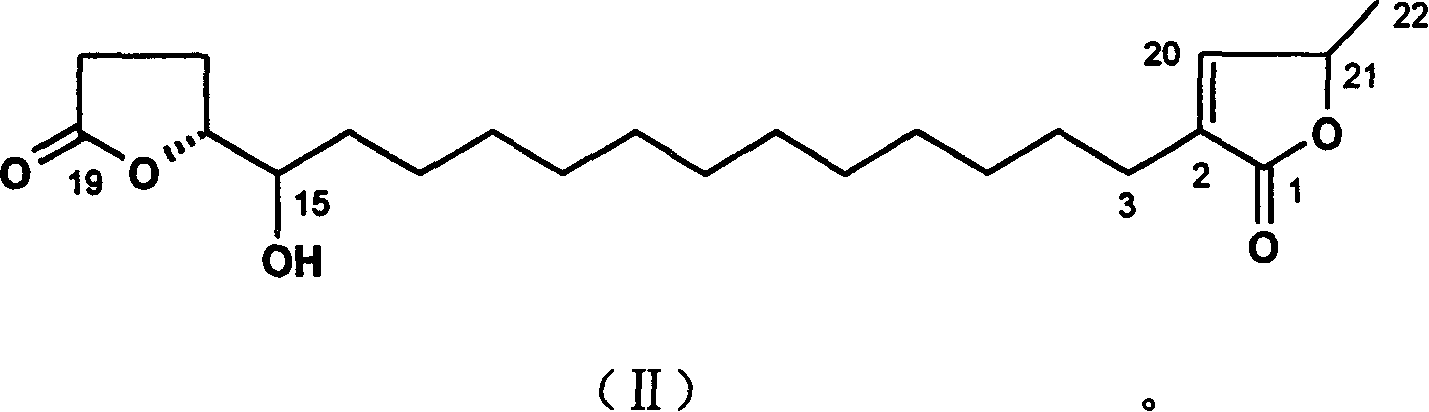
Image
Examples
Embodiment 1
[0024] Embodiment one: the extraction and the separation of anemone diesterin
[0025] The dry powder of custard apple seeds (1 kg) is soaked in 95% ethanol three times, each time for 2 days, and the ethanol extract is concentrated to dryness under reduced pressure to obtain a syrupy ethanol extract. Dissolve the ethanol extract in 300mL 80% dilute ethanol, extract three times with petroleum ether, 100mL each time, wash the petroleum ether extract three times with 80% ethanol, 100mL each time, and merge the washing liquid into the dilute ethanol solution after petroleum ether extraction middle. After the dilute ethanol solution was evaporated to remove ethanol under reduced pressure, water was added to 200 mL to form an aqueous suspension. The aqueous suspension was extracted three times with 100 mL of chloroform. The chloroform extract was dried over anhydrous sodium sulfate and then concentrated to dryness to obtain a brown oil (brown solid at room temperature), which was ...
Embodiment 2
[0027] Embodiment two: structural identification
[0028] Annona diacetin: white needle-like crystals, melting point: 94-96°C; [α] D 24 -3.3° (c0.122, acetone). EI-MSm / z(%): 381[M+H] + (9), 362 [M-H 2 O] + (70), 344(13), 334(8), 295(98), 277(12), 251(18), 112(65), 111(51), 95(78), 85(100), major The interpretation of the fragment ion peaks is given in Formula III. 1 H NMR (CDCl 3 , 400Mz): See Table 1. 13 C NMR (CDCl 3 , 100Mz): See Table 1.
[0029]
[0030] No. δ H (J in Hz) δ C
Embodiment 3
[0031] Example Three: Cytotoxicity to Human Cancer Cells
[0032] Human cancer cell lines: human liver cancer cell line (Bel-7402) and human nasopharyngeal carcinoma cell line (CNE2).
[0033] Main reagents and instruments: RPMI~1640 culture medium, thiazole blue powder (MTT), cisplatin (DDP), enzyme-linked detector
[0034] Test method: use thiazolium blue reduction method (MTT method). The specific method is: under sterile conditions, take the above-mentioned human cancer cells, and adjust them into 1 ≅ 105 / ml The cell suspension was distributed in 96-well cell culture plate, 0.2ml per hole, no drug control group, DMSO solvent control group, known anticancer drug DDP group and 5~6 test substance groups with different concentrations were set up. Set up 4 parallel wells in each group, add drugs to act for 48 hours, measure the OD value of each well on the enzyme-linked analyzer, and calculate according to the fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com