Pharmaceutical composition
A composition and drug technology, applied in the direction of drug combination, drug delivery, active ingredients of heterocyclic compounds, etc., can solve problems such as incompatibility of preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
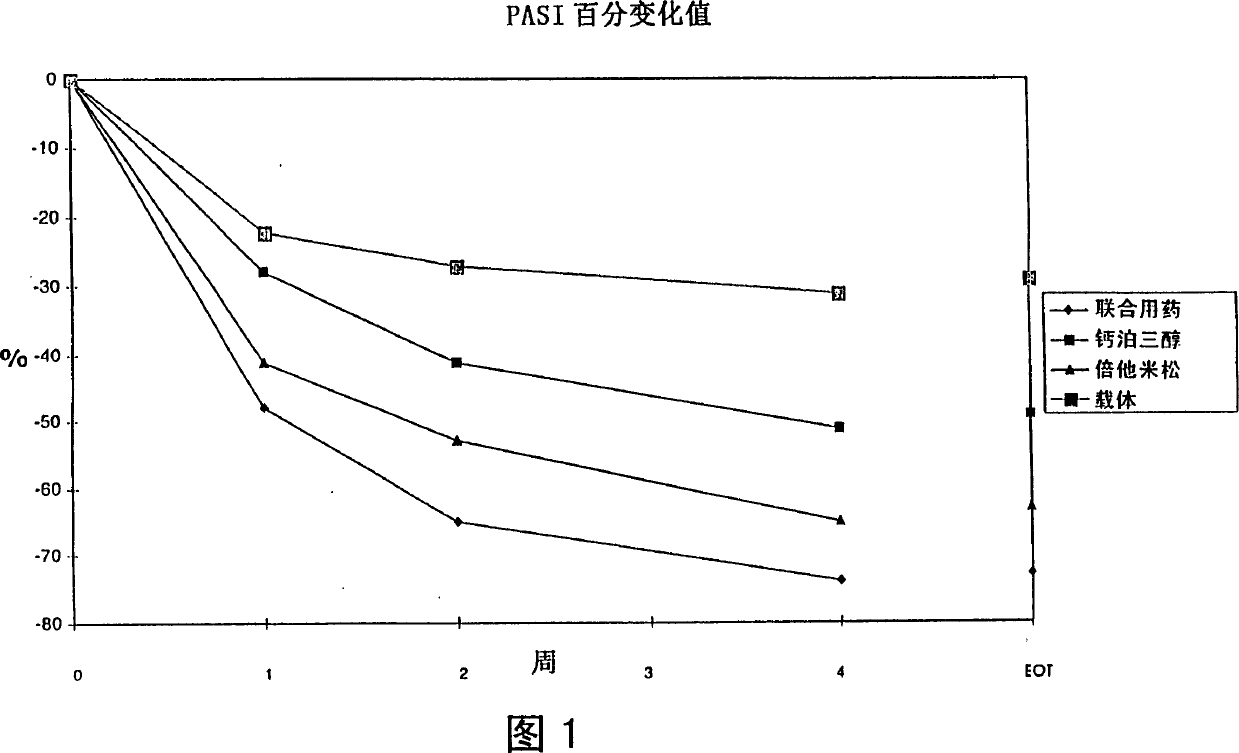
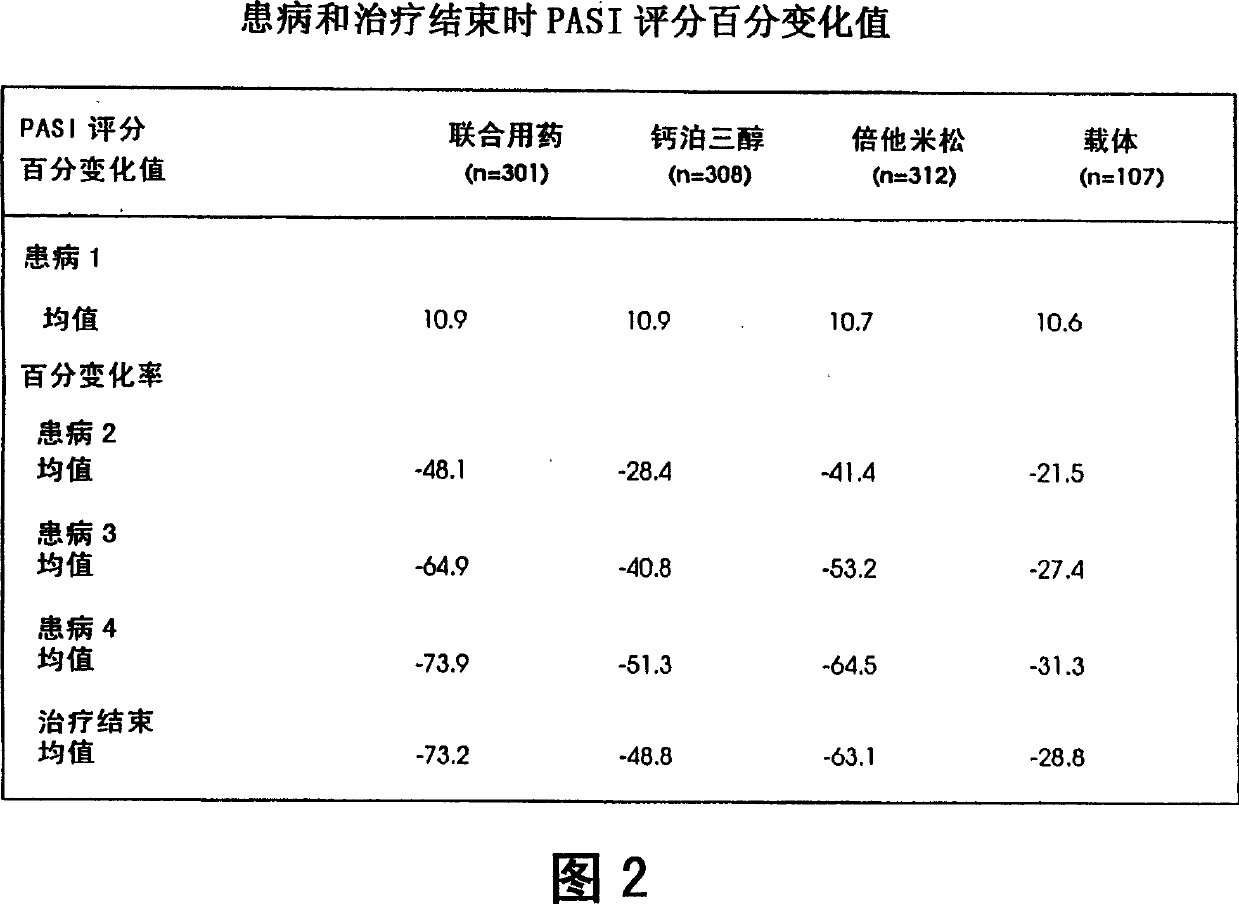
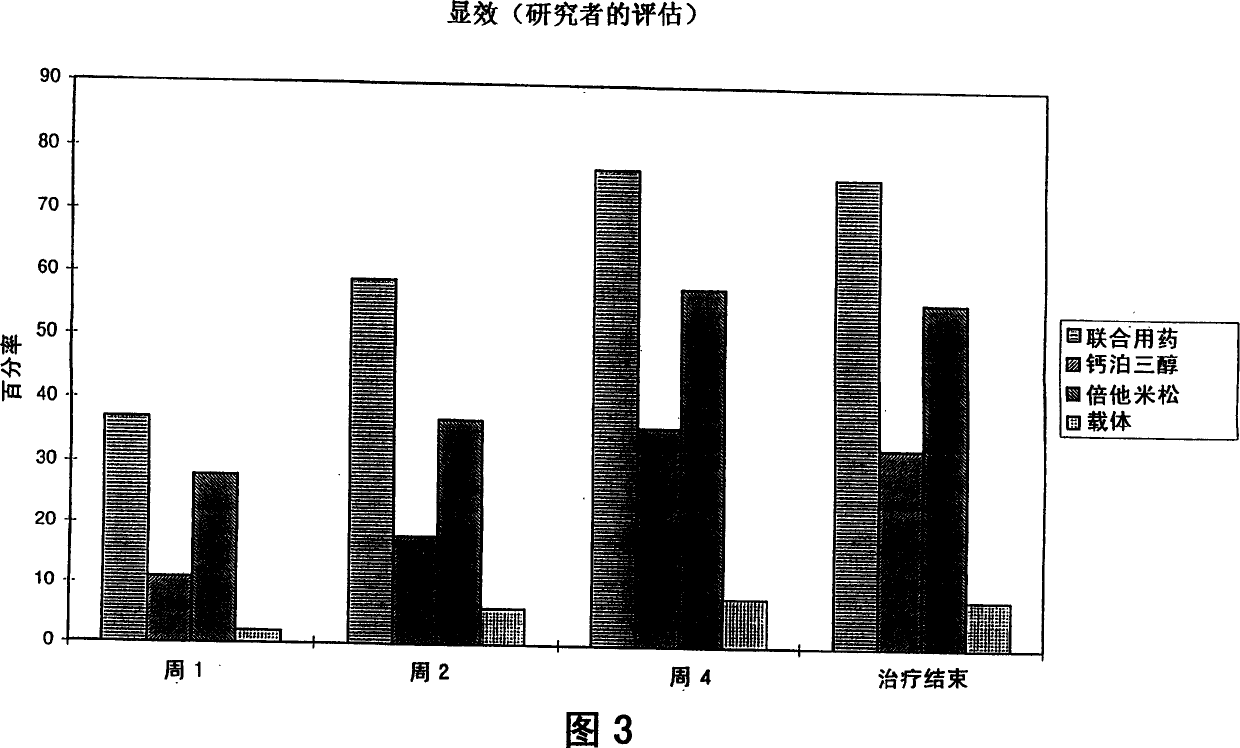
Image
Examples
example
[0011] Examples include:
[0012] alphacalcidol;
[0013] 1α-hydroxy-vitamin D2;
[0014] 1α-hydroxy-vitamin D5;
[0015] 1(S), 3(R)-Dihydroxy-20(R)-(5-Ethyl-5-hydroxy-1-heptyl)-9,10-Danpregna-5(Z), 7(E ), 10(19)-triene;
[0016] 1(S), 3(R)-Dihydroxy-20(R)-(6-Hydroxy-6-methyl-1-heptyl)-9,10-Danpregna-5(2), 7(E )10(19)-triene;
[0017] 1(S), 3(R)-Dihydroxy-20(R)-(6-Hydroxy-6-methylhept-1(E)-en-1-yl-9,10)-Danpregna-5 (Z), 7(E), 10(19)-triene;
[0018] 1(S), 3(R)-dihydroxy-20(R)-(6-ethyl-6-hydroxyl-1-octyl)-9, 10)-pregna-5(Z), 7( E), 10(19)-triene;
[0019] 1(S), 3(R)-dihydroxy-20(R)-(7-hydroxy-7-methyl-1-octyl)-9, 10)-dihydroxy-5(2), 7( E), 10(19)-triene;
[0020] 1(S), 3(R)-Dihydroxy-20(R)-(7-Hydroxy-7-methyloct-1(E)-en-1-yl-9,10)-Danpregna-5 (Z), 7(E), 10(19)-triene;
[0021] 1(S), 3(R)-Dihydroxy-20(R)-(6'-Methyl-1'-heptyl)-9,10-Seppregna-5(Z), 7(E), 10(19)-triene;
[0022] 1(S), 3(R)-dihydroxy-20(S)-(5'-hydroxy-5'-methyl-1'-hexyloxy)-9,10-dihydroxy-5(Z) , 7(E),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com