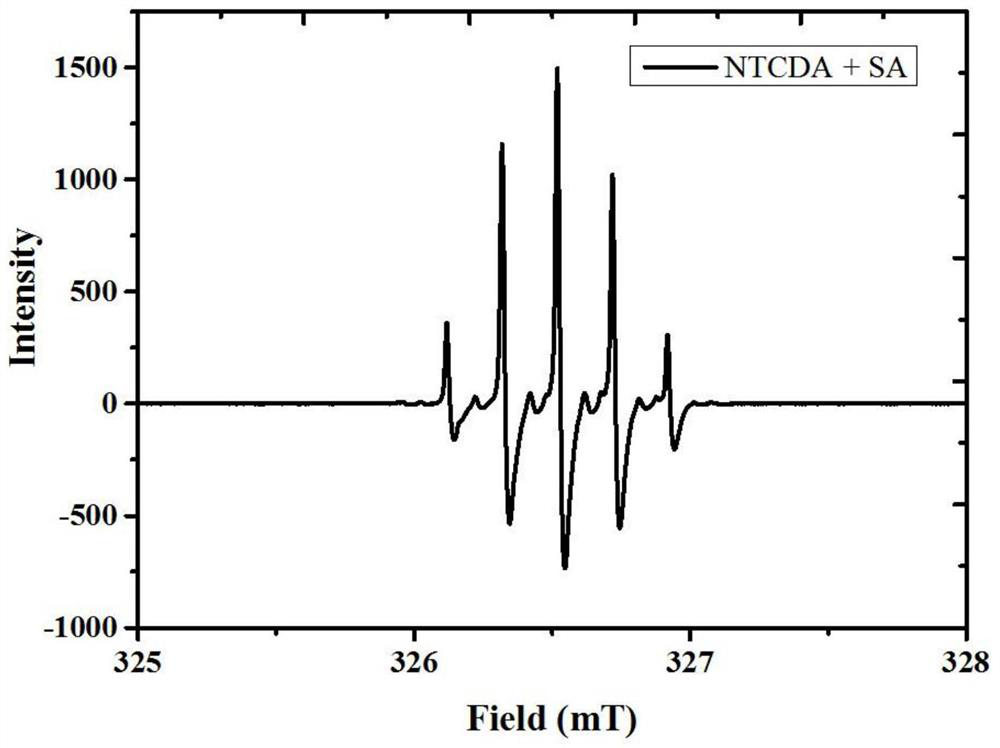
Iodine-regulated reversible-inactivated free radical polymerization catalytic polymerization system
A technology of polymerization catalysis and free radicals, applied in the field of controllable synthesis of polymers, can solve the problems of non-conformity with green chemistry, difficult synthesis, and limitation of practical application, and achieve the effect of controllable molecular weight distribution and great application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
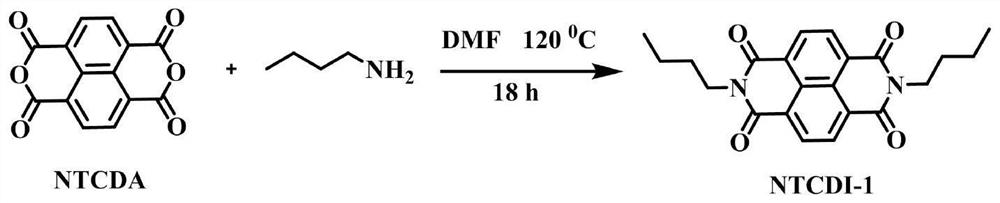
[0071] Example 1 Synthesis of catalyst precursor NTCDI-1
[0072] According to the attached image 3 The shown reaction scheme prepares the appendix figure 1 The NTCDI-1 electron-deficient precursor shown in . Specifically, 1.6 g of 1,4,5,8-naphthalenetetracarboxylic dianhydride (NTCDA) and 100 mL of DMF were added to a 250 mL three-necked flask and placed in an oil bath. Subsequently, argon gas was passed through the reaction solution and heated to 80°C to dissolve completely, then 1.75 g of n-butylamine was slowly added using a constant pressure funnel, the orange solution became cloudy, and then the reaction was refluxed at 120°C for 18 hours to complete the reaction. When the reaction solution was cooled to room temperature, a precipitate formed at the bottom, which was filtered and repeatedly washed with ice water. Finally, the obtained solid was dried in a freeze dryer to obtain a pink product NTCDI-1, which was 1 HNMR spectrum as attached Image 6 shown.
Embodiment 2
[0073] Example 2 Synthesis of catalyst precursor BTI-B
[0074] According to the attached Figure 4 The shown reaction scheme prepares the appendix figure 1 The BTI-B electron-deficient precursor shown in . Specifically, 14.2 g of dimethyl butynedioate was added to a three-necked flask and 120 mL of ethanol was added to dissolve. Subsequently, 42.83 g of benzylamine was slowly added through a constant pressure dropping funnel under ice-water bath conditions, then the constant pressure funnel was rinsed with 20 mL of ethanol and added to the reaction flask, stirred at room temperature for 96 hours, resulting in a large amount of yellow solids, filtered and added with a large amount of ethanol Washed to obtain pure maleimide derivative X-1, 1 H NMR spectrum as attached Figure 7 shown. Subsequently, 3 g of maleimide derivative X-1 was added to a clean three-necked flask, and 30 mL of a mixture of acetic acid and water (V 乙酸 / V 水= 4 / 1). The reaction solution was then heat...
Embodiment 3
[0075] Example 3 Synthesis of catalyst precursor TPT-1
[0076] According to the attached Figure 5 The shown reaction scheme prepares the appendix figure 1 The TPT-1 electron-deficient precursor shown in . Specifically, 1 g of 2,4,6-tris(4-pyridyl)-1,3,5-triazine (TPT) and 25 mL of DMF were added to a clean three-necked flask and placed in an oil bath under argon. Heating under conditions, then adding 6.55mL 1-iodobutane to carry out reflux reaction at 120 ° C, the color of the solution changed from a white suspension to brownish purple, and finally changed to dark red black, the reaction was terminated after 24 hours of reaction and cooled to room temperature. Then, the reaction solution was precipitated into a large amount of n-hexane, washed repeatedly with stirring, and the upper layer of n-hexane containing a large amount of DMF was poured off. After repeating this operation for many times, the DMF solvent and the residual unreacted butyl chain on the TPT were removed...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com