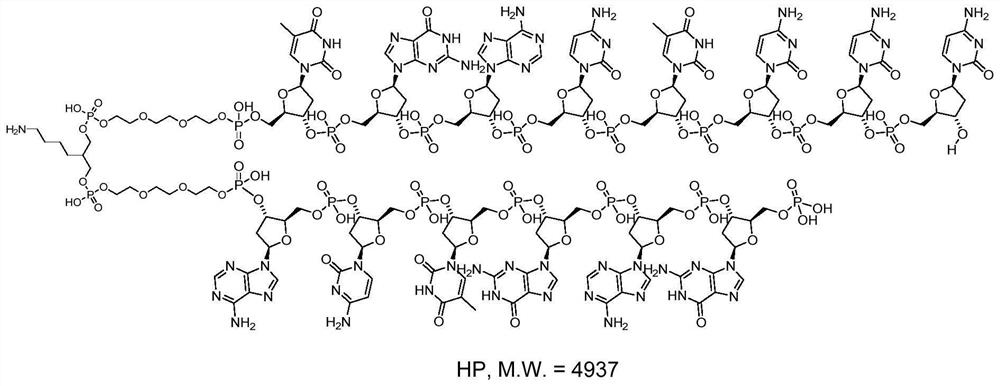
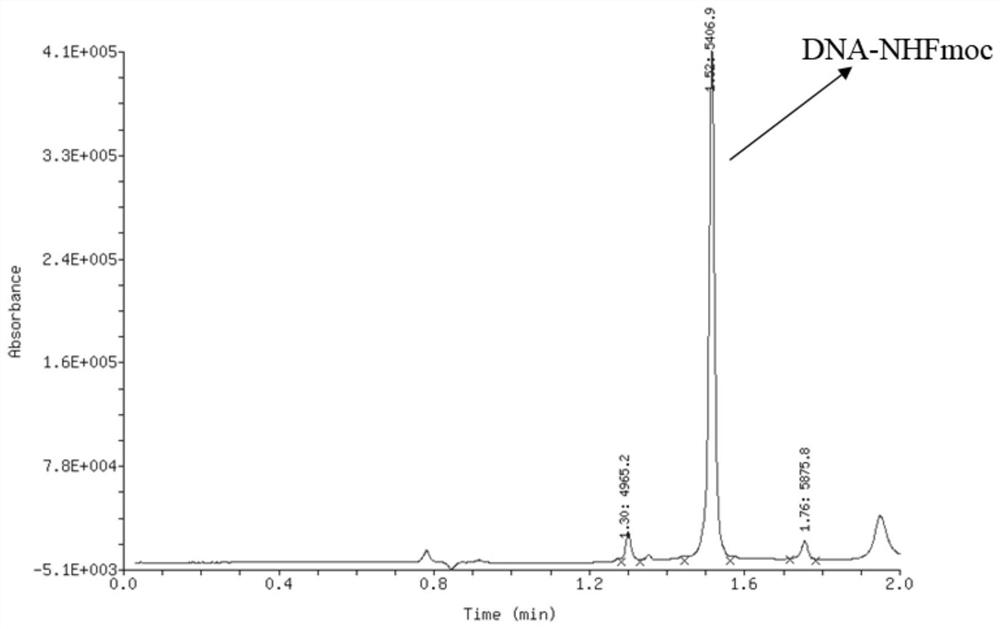
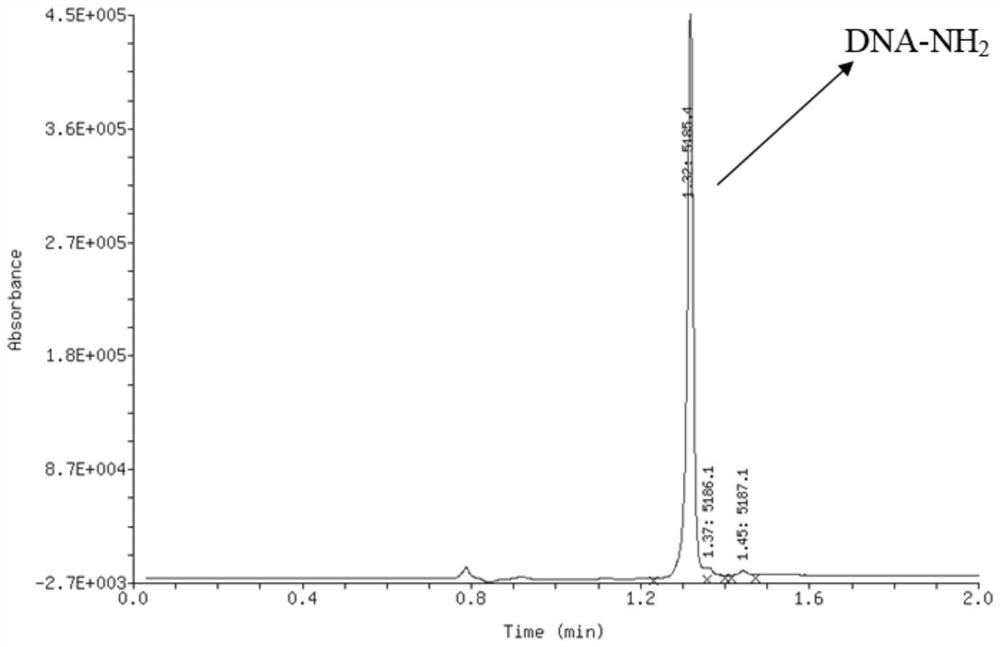
Oligonucleotide-disulfide and synthesis method thereof
An oligonucleotide and disulfide technology, applied in the field of oligonucleotide-disulfide and its synthesis, can solve the problems of DNA instability, influence chemical reaction, denaturation, etc., and achieve good DNA integrity, The effect of high conversion rate and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0196] Example 1. Synthesis of oligonucleotide-disulfide P1
[0197]
[0198] 1-Propanethiol S2-1 (20 mmol / L tetrahydrofuran solution) was added to an aqueous solution of 1 nanomolar oligonucleotide-thiol compound S1-1 (1 mmol / L, 1 equiv, 1 μL). , 50 equiv, 2.5 μL) and 1,1,3,3-tetramethylguanidine (TMG, 4 mmol / L tetrahydrofuran solution, 10 equiv, 2.5 μL), the mixture was mixed well by vortexing After reaction at 25°C for 10 minutes. After the reaction was completed, 10% of the total volume of 5 mol / L sodium chloride solution and 3 times the volume of absolute ethanol were added to the reaction solution. High-speed refrigerated centrifugation (4° C., 12,000 rpm, 15 minutes), the supernatant was discarded, and the precipitate was oligonucleotide-disulfide P1. The remaining precipitate was dissolved in deionized water to prepare an aqueous solution of oligonucleotide-disulfide P1 with a concentration of 1 mmol / L. Detected by liquid chromatography-mass spectrometry, the mol...
Embodiment 2
[0200] Example 2. Synthesis of oligonucleotide-disulfide P4
[0201]
[0202] 3-Methoxythiophenol S2-4 (80 mmol / L) was added to an aqueous solution of 1 nanomolar oligonucleotide-thiol compound S1-1 (1 mmol / L, 1 equiv, 1 μL). L tetrahydrofuran solution, 200 equiv, 2.5 μL) and 1,1,3,3-tetramethylguanidine (TMG, 4 mmol / L tetrahydrofuran solution, 10 equiv, 2.5 μL), the mixture was vortexed After thorough mixing, the reaction was carried out at 25°C for 1 hour. After the reaction was completed, 10% of the total volume of 5 mol / L sodium chloride solution and 3 times the volume of absolute ethanol were added to the reaction solution. High-speed refrigerated centrifugation (4° C., 12,000 rpm, 15 minutes), the supernatant was discarded, and the precipitate was oligonucleotide-disulfide P4. The remaining precipitate was dissolved in deionized water to prepare an oligonucleotide-disulfide P4 aqueous solution with a concentration of 1 mmol / L. Detected by liquid chromatography-mass...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com