Application of isoliquiritin in preparation of medicine for treating osteoporosis
A technology for isoliquiritigenin and osteoporosis, which is applied in the application field of isoliquiritigenin in the preparation of drugs for treating osteoporosis, can solve the problems of troublesome access, high price, side effects and the like, and achieves inhibiting the formation of osteoporosis. , cheap, clear ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1: Animal experiment
[0013] The steps used in this experiment are: Step 1: Select 30 8-week-old mice and divide them into sham operation group (sham), castration group (OVX), castration group + low concentration group (10mg / kg), castration group Group + medium concentration group (30mg / kg), castration group + high concentration group (50mg / kg), each group has 6 mice to establish a mouse osteoporosis model; Step 2: Isoliquiritigenin is dissolved in carboxymethyl fiber CMC-Na, 0.5 ml of isoliquiritigenin solution was injected into the latter three groups of mice by gavage, and the mice were reared in the same rearing environment. The condition of osteoporosis was judged, and the experimental mice were given continuous gavage for one month. The condition of the osteoporosis in the mice was preliminarily analyzed by micro tomography (Micro CT), and the mice were examined by histopathological examination (HE staining). Slice the affected area to detect the morphol...
Embodiment 2
[0014] Embodiment 2: CCK-8 detects the drug toxicity test of isoliquiritin
[0015] The steps used in this test are:
[0016] Step 1: The configuration of isoliquiritigenin, from the purchased isoliquiritigenin (HPLC>98), weigh 20 mg, use 1 ml of DMSO to prepare 20 mg / ml, and then dilute it with medium to different concentrations of working solution;
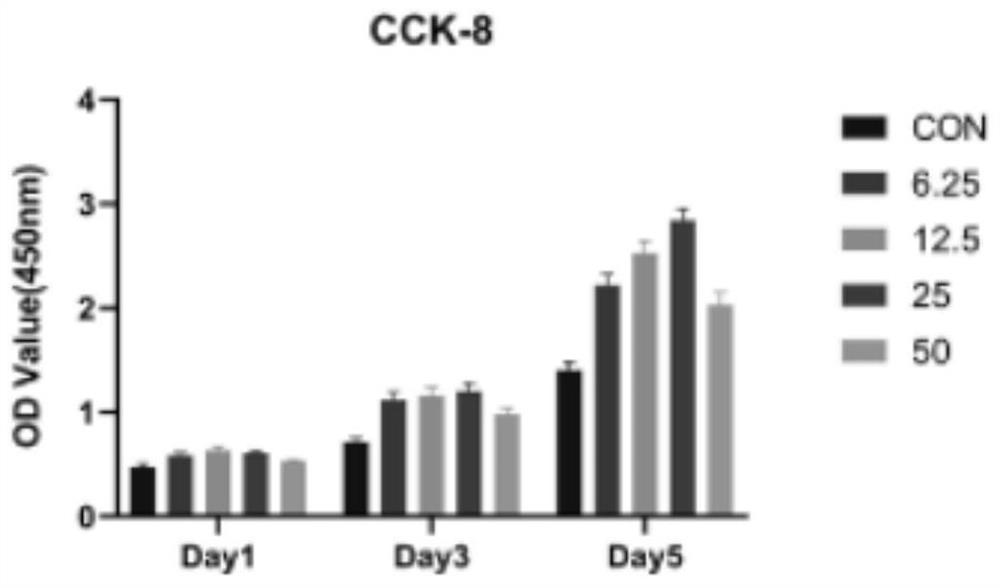
[0017] Step 2: Detect the effect of isoliquiritigenin on the proliferation of bone marrow mesenchymal stem cells in vivo, inoculate bone marrow mesenchymal stem cells in 896-well plates at a cell density of 3000 cells / well, and add different concentrations of isoliquiritigenin for stimulation after 24 hours , the concentrations were 6.25, 12.5, 25, and 50, respectively, cultured at 37°, 5% CO2 for 1, 3, and 5 days, and detected with CCK-8.
[0018] Test results such as figure 1 and 2 As shown in the figure, it can be seen from the figure that isoliquiritigenin has a certain promoting effect on cell growth. When the concentrat...
Embodiment 3
[0019] Example 3: In vitro experiments
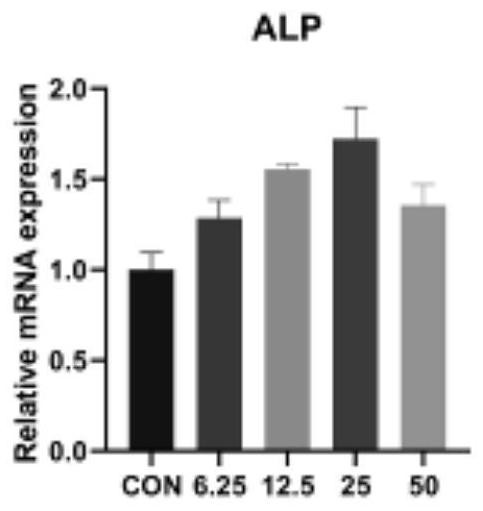
[0020] The steps used in this experiment are: step 1: obtain and culture bone marrow mesenchymal stem cells; step 2: determine the optimal drug concentration of isoliquiritin according to the results obtained in Example 2; step 3: conduct an osteogenic induction test, MAPK Inhibitors and autophagy (3-MA) inhibitors inhibited related expressions, and then used Western blotting, immunofluorescence staining, alkaline phosphatase staining and quantification, and real-time quantitative PCR (Real-timeqPCR). ), Alizarin red staining was used for verification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com