Preparation method of peramivir key intermediate
An intermediate and key technology, applied in the direction of organic chemistry, can solve the problems of low production yield, many steps, consumption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
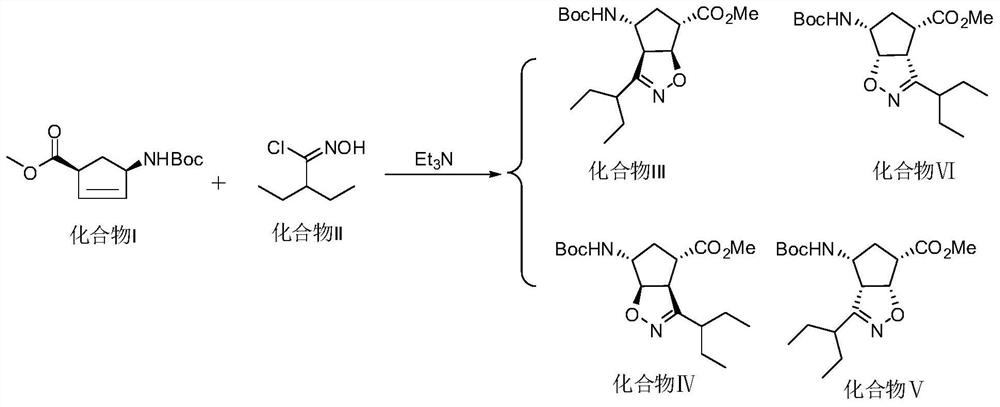
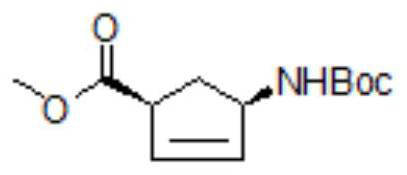
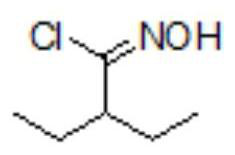
[0067] Step 1: get 90g (0.43mol) of (1S,4R)-(-)-[[(1,1-dimethylethoxy)carbonyl]amino]cyclopent-2-ene-1-carboxylic acid methyl Ester, 600g toluene and 130g (1.28mol) triethylamine were added to the reaction flask, mixed and heated to 60-70°C to obtain a mixed solution, and the previously prepared 2-ethyl-N-hydroxybutanimine was added to the mixed solution. The toluene solution of the acid chloride was subjected to cyclization reaction. After 3 hours, the temperature of the mixed solution in the reaction flask was cooled to room temperature and 200 g of water was added. The organic layer is removed;
[0068] Step 2: add 400g of n-heptane to the product after the cyclization reaction described in step 1 and keep stirring at a temperature of 60-70 ° C until it is dissolved, add 4.5g of activated carbon to the product after the dissolution and decolorize for 0.5h, after the decolorization The product was filtered for the first time, cooled to 0-5 °C for stirring, filtered for the ...
Embodiment 2
[0071] Step 1: get 30g (0.14mol) of (1S,4R)-(-)-[[(1,1-dimethylethoxy)carbonyl]amino]cyclopent-2-ene-1-carboxylic acid methyl Ester, 200g of toluene and 43.3g (0.43mol) of triethylamine were added to the reaction flask, mixed and heated to 60-70°C to obtain a mixed solution, and into the mixed solution was added the 2-ethyl-N-hydroxybutanimine prepared above. The toluene solution of the acid chloride was subjected to cyclization reaction. After 3 hours, the temperature of the mixed solution in the reaction flask was cooled to room temperature and 70 g of water was added. The organic layer is removed;
[0072] Step 2: add 140g of n-heptane to the product after the cyclization reaction described in step 1 and keep stirring at a temperature of 60-70 ° C until it is dissolved, add 1.5g of activated carbon to the product after the dissolution and decolorize for 0.5h, after decolorization The product was filtered for the first time, cooled to 0-5 °C for stirring, filtered for the s...
Embodiment 3
[0075] Step 1: get 30g (0.14mol) of (1S,4R)-(-)-[[(1,1-dimethylethoxy)carbonyl]amino]cyclopent-2-ene-1-carboxylic acid methyl Ester, 200g of toluene and 43.3g (0.43mol) of triethylamine were added to the reaction flask, mixed and heated to 60-70°C to obtain a mixed solution, and into the mixed solution was added the 2-ethyl-N-hydroxybutanimine prepared above. The toluene solution of the acid chloride was subjected to cyclization reaction. After 3 hours, the temperature of the mixed solution in the reaction flask was cooled to room temperature and 70 g of water was added. The organic layer is removed;
[0076] Step 2: add 140g of n-heptane to the product after the cyclization reaction described in step 1 and keep stirring at a temperature of 60-70 ° C until it is dissolved, add 1.5g of activated carbon to the product after the dissolution and decolorize for 0.5h, after decolorization The product was filtered for the first time, cooled to 0-5 °C for stirring, filtered for the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com