Application of spiro piperazine quaternary ammonium salt derivative in preparation of medicine for treating irritable bowel syndrome
A technology of irritable bowel syndrome and medicine, which is applied in the field of medicine, can solve the problems such as inability to obtain curative effect, and achieve the effect of obvious symptoms of diarrhea and constipation, wide application range, and relief of symptoms of diarrhea and constipation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Therapeutic effect of the compound of Example 1 on diarrhea-induced irritable bowel syndrome (D-IBS) rats constructed by drugs
[0036] 1. Materials and methods
[0037] 1.1 Experimental animals
[0038] SD male rats, weighing 180-220 g.
[0039] 1.2 Building the model
[0040] The rats were given senna by gavage at 8:00 in the morning, at a dose of 10 mL / kg, once a day, for 4 consecutive weeks, and for the second week, the rats were irritated with wooden clips by clipping their tails. Its fights for 30min a day. Modeling was stopped from the 5th week.
[0041] 1.3 Grouping and Drugs
[0042] Blank group: 8 normal rats that did not participate in the modeling were administered with normal saline by gavage, according to the body weight of 20 mL / kg;
[0043] Model group: 8 model rats, intragastric administration of normal saline, 20 mL / kg of body weight;
[0044] Positive control group: 8 model rats, intragastric administration of 1 mg / mL pinaverium bromide aqueous s...
Embodiment 2
[0067] Example 2 Therapeutic effect of compound on diarrhea-type irritable bowel syndrome (D-IBS) rats constructed by psychological stress method
[0068] 1. Materials and methods
[0069] 1.1 Experimental animals
[0070] SD male rats, weighing 180-220 g.
[0071] 1.2 Building the model
[0072] The IBS rat model was induced by chronic restraint tail-pinch stimulation. For every 5 rats in the same cage, use sponge-coated clips to clamp the proximal 1 / 3 of the rat's tail. Be careful not to damage the skin, make it irritated, and fight with other rats, so as to provoke the whole cage of rats to fight. Observe Rats were stimulated by irritating stress response (groaning, standing confrontation, writhing, screaming, biting, etc.) for 30 minutes. One hour after the end of the tail clipping stimulation, the rats were anesthetized with 30% chloral hydrate (3 mL / kg), and the front shoulders, front upper limbs and chest were bound with paper tape to restrain them from scratching t...
Embodiment 3
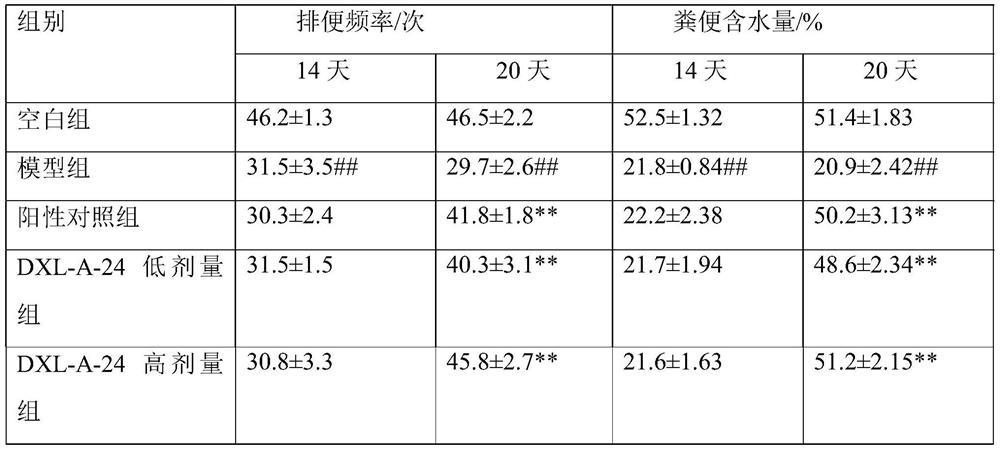
[0101] Example 3 Therapeutic effect of compound DXL-A-24 on constipation-predominant irritable bowel syndrome (C-IBS) rats
[0102] 1. Materials and methods
[0103] 1.1 Experimental animals
[0104] SD rats, half male and half female, weighing 180-220 g.
[0105] 1.2 Building the model
[0106] The C-IBS model was established by gavage with ice water. The rats were raised in a single cage and were given 2 mL of normal saline at 0-4°C by gavage every day. The model was established for 14 days and observed. Decreased stool water content.
[0107] 1.3 Grouping and administration
[0108] Blank group: 8 normal rats that did not participate in the modeling were administered with normal saline by gavage, according to the body weight of 20 mL / kg;
[0109]Model group: 8 model rats, intragastric administration of normal saline, 20 mL / kg of body weight;
[0110] Positive control group: 8 model rats, intragastric administration of 0.4 mg / mL cisapride suspension, according to body ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com