Monolithic non-noble metal alloy hydrazine oxidation catalyst as well as preparation method and application thereof
An oxidation catalyst, non-precious metal technology, used in nanotechnology, structural parts, electrical components, etc. for materials and surface science, can solve the problems of little research, active site shielding, reduced catalytic activity, etc. control, easy availability of raw materials, and the effect of improving mass transfer performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
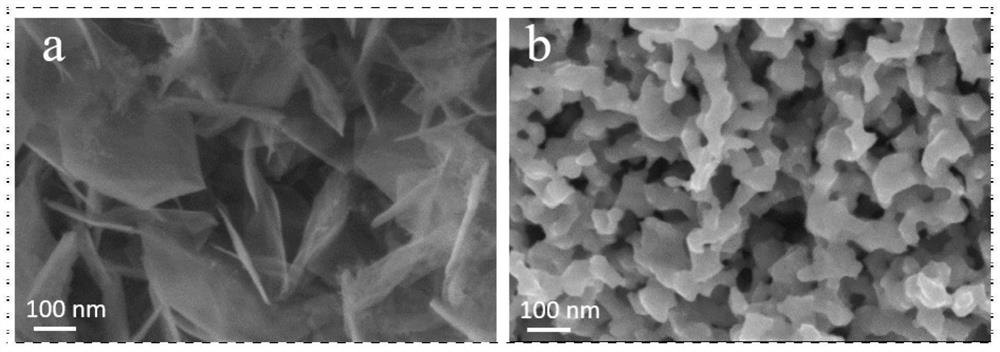
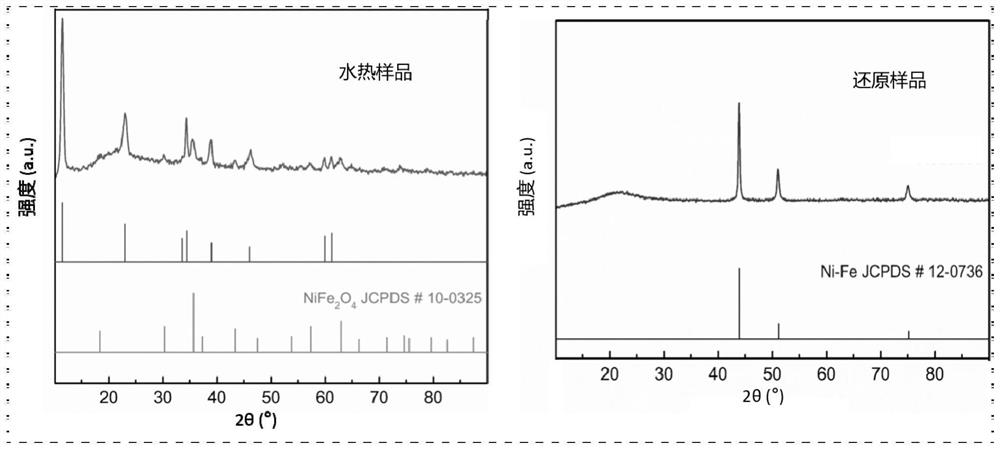
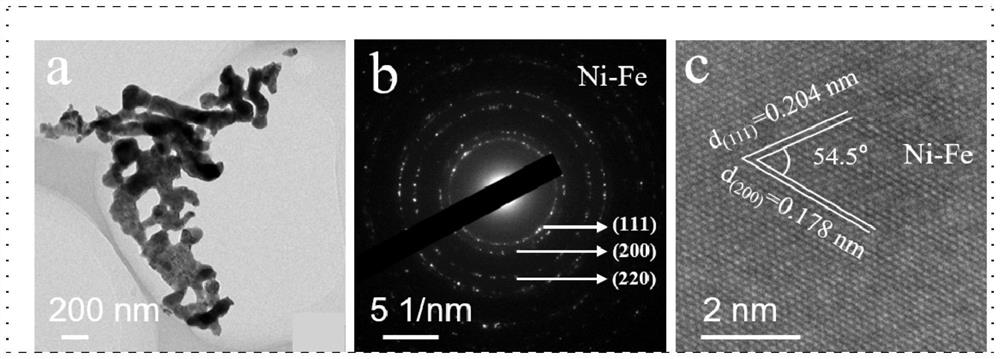
[0043] Preparation process reference Figure 18 .
[0044] With nickel foam as the carrier, its thickness is 1.60mm, and the areal density is ~650g / m 2 , the aperture is 0.20 ~ 0.80mm. Nickel foam (1×4cm 2 ) were ultrasonically cleaned with ethanol, hydrochloric acid solution (3M) and deionized water for 10 minutes in sequence. The Ni (NO 3 ) 2 ·6H 2 O(0.1M), Fe(NO 3 ) 3 ·9H 2 O (0.2M) and urea (0.2M) were dissolved in 36 mL of deionized water. After continuous stirring for 30 minutes, the mixed solution and a piece of cleaned NF were transferred to a stainless steel autoclave lined with PTFE, treated at a constant temperature of 120 °C for 12 hours, and then cooled to room temperature naturally. Vacuum drying was carried out at 60 °C for 12 hours to obtain a hydrothermal sample NiFe(1:2)-CH / NF; then the hydrothermal sample and 30 mL of ethylene glycol were transferred to a stainless steel lined with PTFE In the autoclave, the temperature was raised to 120 °C for 12...
Embodiment 2
[0062] With carbon cloth (CC, 1×4cm 2 ) as a carrier, after 20 minutes of ultrasonic cleaning with hydrochloric acid (1M), absolute ethanol and deionized water in turn, the carbon cloth was subjected to heat treatment at 500 ° C for 3 hours under an air atmosphere, and then placed in a solution containing transition metal salts, precipitation in the hydrothermal kettle of the agent and solvent. The transition metal salts, organic ligands and mixed organic solvents used in the hydrothermal reaction are: Ni(NO 3 ) 2 ·6H 2 O(0.1M), Fe(NO 3 ) 3 ·9H 2 O (0.2M), hexamethylenetetramine (0.2M) and 36mL of deionized water solution, the hydrothermal reaction conditions are 120 ℃ constant temperature for 12 hours, and then naturally cooled to room temperature, the prepared samples were fully washed and then kept at 60 ℃ Under vacuum drying for 12 hours, the hydrothermal sample NiFe-CH / CC was obtained; then the hydrothermal sample and 30 mL of ethylene glycol were transferred to a s...
Embodiment 3
[0071] Using foam iron (FF) as the carrier, its thickness is 1.80mm, and the areal density is 650g / m 2 , the aperture is 0.20 ~ 0.80mm. Foam iron (1×4cm 2 ) were ultrasonically cleaned with ethanol for 10 minutes, activated with 1M hydrochloric acid solution for 5 minutes, and ultrasonically cleaned with deionized water for 10 minutes. The Ni (NO 3 ) 2 ·6H 2 O(0.1M), Fe(NO 3 ) 3 ·9H 2 O (0.2M) and urea (0.2M) were dissolved in 36 mL of deionized aqueous solution, and after continuous stirring for 30 minutes, the mixed solution and a piece of clean CF were transferred to a Teflon-lined stainless steel autoclave, and the mixture was heated for 120 After being treated at a constant temperature for 12 hours, it was naturally cooled to room temperature. The prepared sample was fully cleaned and then vacuum-dried at room temperature for 12 hours to obtain a hydrothermal sample NiFe-CH / FF; then the hydrothermal sample and 30 mL of ethylene glycol were transferred. In a stainl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com