Method for synthesizing 2, 3-disubstituted indanone derivative in water phase at one time
A one-time, two-substitution technology, applied in the direction of condensation to prepare carbonyl compounds, organic chemistry, etc., can solve the problem of limited application of disubstituted olefin substrates, and achieve the effects of wide expansion range, low toxicity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] [RhCl(cod)] 2 Catalyze the reaction of diphenylacetylene and 2-formylphenylboronic acid, the process is as follows:
[0030] Under nitrogen protection, [RhCl(cod)] was added sequentially in the reaction flask 2 5mol%, 0.15mmol of diphenylacetylene, 0.225mmol of 2-formylphenylboronic acid, 45μl of triethylamine, 0.5ml of water, and stirred at 50°C for 16h to obtain 42.1mg of the product with an isolated yield of 98%.
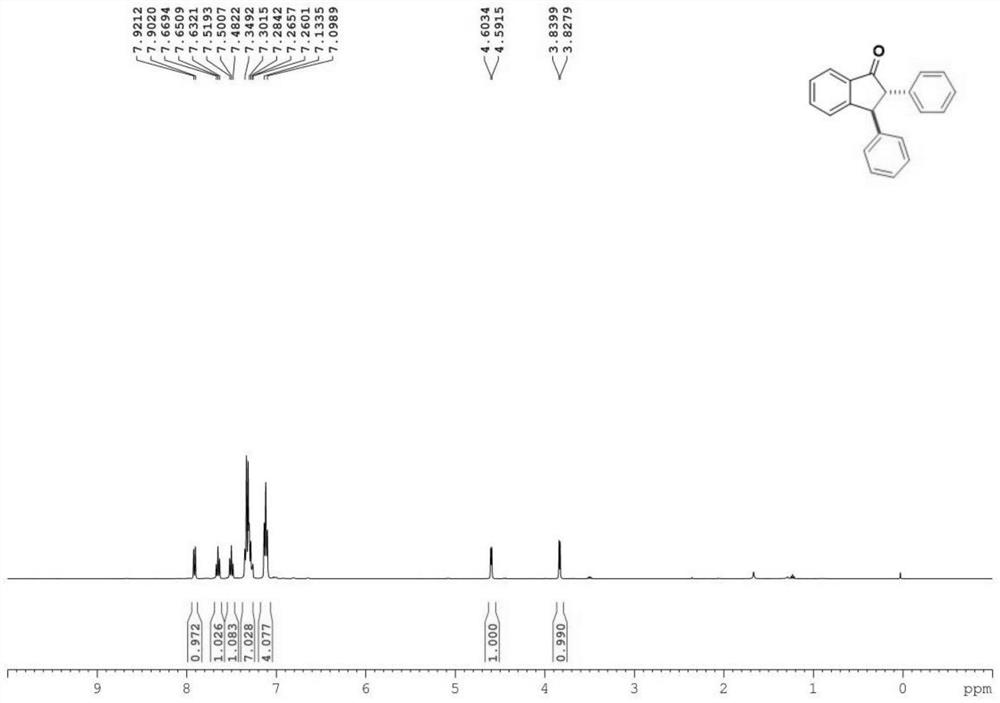
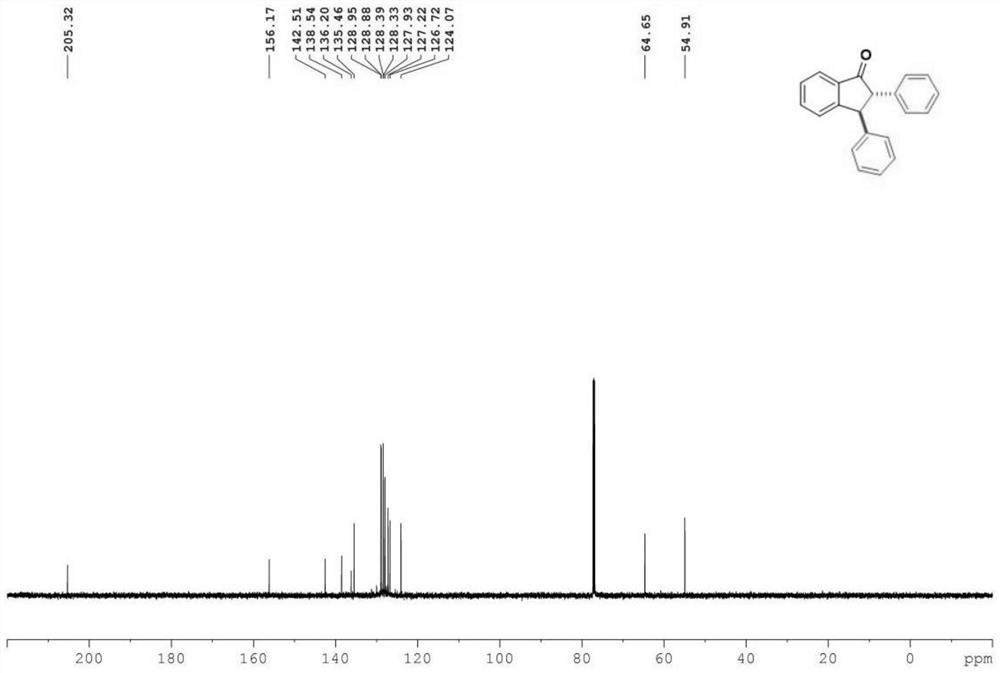
[0031] Characterize the product, the result is: 1 H NMR (400MHz, Chloroform-d) δ7.91(d, J=7.7Hz, 1H), 7.65(t, J=7.5Hz, 1H), 7.50(t, J=7.5Hz, 1H), 7.31(m ,J=12.1,7.0Hz,7H),7.12(t,J=7.0Hz,4H),4.60(d,J=4.8Hz,1H),3.83(d,J=4.8Hz,1H). 13 CNMR (151MHz, Chloroform-d) δ205.3, 156.2, 142.5, 138.5, 136.2, 135.5, 129.0, 128.9, 128.4, 128.3, 127.9, 127.2 (d, J=2.0Hz), 126.7, 124.1, 64.7, 54.9.
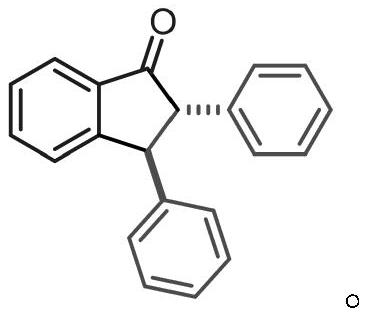
[0032] The product structure is:
[0033]
Embodiment 2
[0035] [RhCl(cod)] 2Catalyze the reaction of 1,2-bis(4-methylphenyl)acetylene with 2-formylphenylboronic acid, the process is as follows:
[0036] Under nitrogen protection, [RhCl(cod)] was added sequentially in the reaction flask 2 5mol%, 1,2-bis(4-methylphenyl)acetylene 0.15mmol, 2-formylphenylboronic acid 0.225mmol, triethylamine 45μl, water 0.5ml, stirred at 50°C for 16h to obtain 35.1mg of the product, separated Yield 75%.
[0037] Characterize the product, the result is: 1 H NMR (400MHz, Chloroform-d) δ7.89(d, J=7.7Hz, 1H), 7.63(t, J=7.5Hz, 1H), 7.48(t, J=7.4Hz, 1H), 7.31(d ,J=7.7Hz,1H),7.13(dd,J=8.1,2.3Hz,4H),7.00(dd,J=7.8,5.6Hz,4H),4.53(d,J=4.8Hz,1H),3.77 (d,J=4.8Hz,1H),2.34(d,J=4.4Hz,6H). 13 C NMR (151MHz, Chloroform-d) δ205.6, 156.4, 139.6, 136.8, 136.2, 135.6, 135.3, 129.6 (d, J=4.7Hz), 128.3, 128.2, 127.8, 126.7, 124.0, 64.4, 54.6, 21.1 (d ,J=5.6Hz).
[0038] The product structure is:
[0039]
Embodiment 3
[0041] [RhCl(cod)] 2 Catalyze the reaction of 1,2-bis(4-fluorophenyl)acetylene with 2-formylphenylboronic acid, the process is as follows:
[0042] Under nitrogen protection, [RhCl(cod)] was added sequentially in the reaction flask 2 5mol%, 1,2-bis(4-fluorophenyl)acetylene 0.15mmol, 2-formylphenylboronic acid 0.225mmol, triethylamine 45μl, water 0.5ml, stirred at 50°C for 16h to obtain 46.3mg of the product, isolated The rate is 96%.
[0043] Characterize the product, the result is: 1 H NMR (400MHz, Chloroform-d) δ7.89(d, J=7.7Hz, 1H), 7.66(t, J=7.5Hz, 1H), 7.51(t, J=7.5Hz, 1H), 7.29(d ,J=7.7Hz,1H),7.19-6.91(m,8H),4.50(d,J=5.2Hz,1H),3.73(d,J=5.2Hz,1H). 13 C NMR (101MHz, Chloroform-d) δ204.9, 162.2(d, J=247.4Hz), 162.1(d, J=247.1Hz), 155.6, 137.9(d, J=3.5Hz), 136.0, 135.8, 133.9(d ,J=3.5Hz),130.1(d,J=8.2Hz),129.5(d,J=8.3Hz),128.7,126.6,124.2,115.99(d,J=21.7Hz),115.98(d,J=21.6 Hz), 64.2, 54.4.
[0044] The product structure is:
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com