Tilmicosin premix and preparation method thereof
A technology of tilmicosin and premix, applied in the direction of medical formula, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve low bioavailability, gastric acid damage, loss of appetite, etc. problems, to achieve the effect of improving the treatment effect, simple and easy process, and simple process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
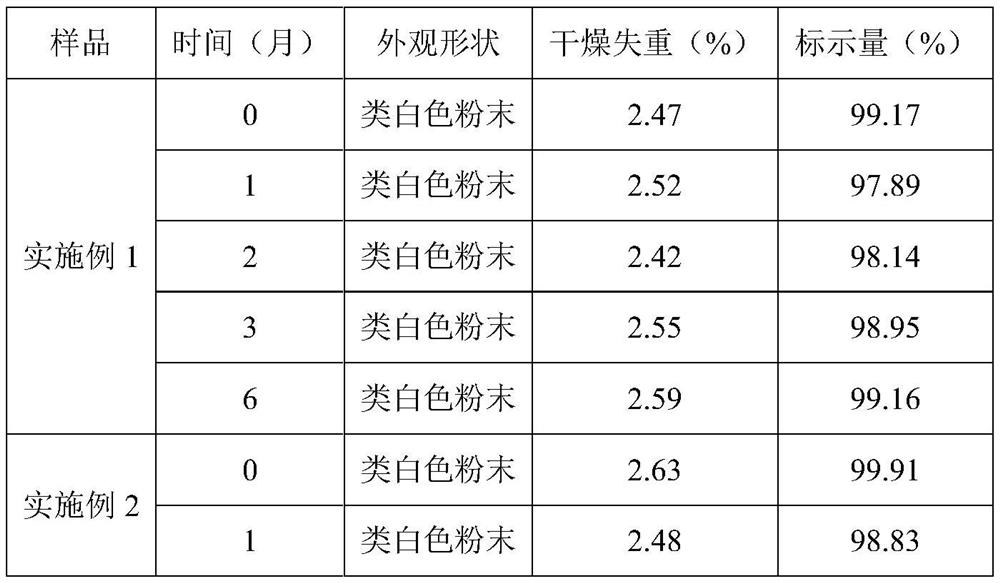
Embodiment 1
[0021] A kind of tilmicosin premix of the present embodiment and preparation method thereof, during preparation, comprises the following steps:
[0022] (1) Take by weighing 10.0% tilmicosin and 1.0% adenosine, stir evenly to obtain mixed powder;
[0023] (2) Take by weighing 20% cellulose acetate phthalate, add water to make adhesive slurry, where the water consumption is 8 times of the quality of the enteric carrier material;
[0024] (3) Add the mixed powder of step (1) to the adhesive slurry, turn on the mixer, fully stir for 30min, and mix evenly;
[0025] 4) Add anhydrous glucose with a mass percentage of 69% to the mixture in step (3), stir well for 20 minutes to make a soft material, granulate, dry at 60°C for 6 hours, and pass through a No. 4 sieve to obtain the product.
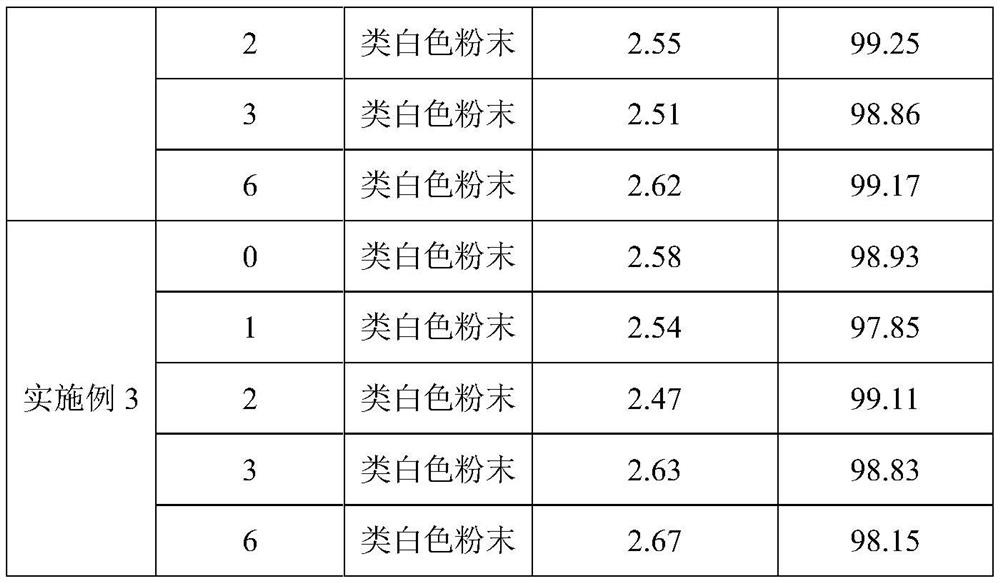
Embodiment 2
[0027] A kind of tilmicosin premix of the present embodiment and preparation method thereof, during preparation, comprises the following steps:
[0028] (1) Take by weighing 20.0% tilmicosin and 2.0% probenecid in mass percentage, stir evenly to obtain mixed powder;
[0029] (2) Take by weighing an acrylic resin with a mass percentage of 35%, add water to make a bonding slurry, where the water consumption is 7 times of the quality of the enteric-coated carrier material;
[0030] (3) Add the mixed powder of step (1) to the adhesive slurry, turn on the mixer, fully stir for 30min, and mix evenly;
[0031] 4) Add lactose with a mass percentage of 43% to the mixture in step (3), stir well for 20 minutes to make a soft material, granulate, dry at 80°C for 4 hours, and pass through a No. 4 sieve to obtain the product.
Embodiment 3
[0033] A kind of tilmicosin premix of the present embodiment and preparation method thereof, during preparation, comprises the following steps:
[0034] (1) Take by weighing 30.0% tilmicosin and 5.0% phosphatidic acid-beta lactoglobulin complex, stir evenly to obtain mixed powder;
[0035] (2) Take carboxymethyl ethyl cellulose with a mass percentage of 50%, add water to make a bonding slurry, where the water consumption is 10 times the quality of the enteric carrier material;
[0036] (3) Add the mixed powder of step (1) to the adhesive slurry, turn on the mixer, fully stir for 30min, and mix evenly;
[0037] (4) Add 5% anhydrous glucose and 10% corn starch to the mixture in step (3), stir well for 20 minutes to make a soft material, granulate, dry at 70°C for 5 hours, and pass through No. 4 Sieve, that is.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com