Application of chelidonine in preparation of medicine for treating acute myelogenous leukemia with FLT3-ITD mutation
A technology of FLT3-ITD and chelidonine, which can be used in drug combinations, pharmaceutical formulations, anti-tumor drugs, etc., can solve problems such as no research on the effect of chelidonine, and achieve the effects of reducing infiltration, prolonging survival time, and increasing expression.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: in vitro experiment
[0042] 1. Chelidonine inhibits the proliferation of FLT3-ITD mutant cell lines
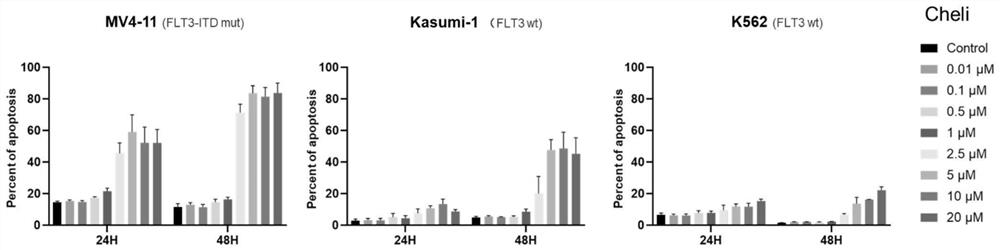
[0043] In order to determine the effect of Chelidonine on the proliferation of cells with FLT3-ITD mutation, we selected the MV4;11 cell line with FLT3-ITD mutation as the research object, and also used two cell lines without FLT3-ITD, K562 and Kasumi-1 as comparison. Eight groups of different concentrations of Chelidonine were used to treat the above three cell lines, and CCK-8 was used to detect cell proliferation. The results are shown in figure 1 .
[0044] According to the test results of CCK-8, the concentration of Chelidonine ≤ 1 μM will not affect the proliferation of cells. However, the concentration of Chelidonine ≥ 2.5 μM can significantly inhibit the proliferation of FLT3-ITD mutant MV4; 11 cell line, and its 24h IC50 is 2.8 μM. The 24h IC50 of the two cell lines without FLT3-related mutations were: K562 4.9μM, Kasumi-1 5.8μM. It is sugge...
Embodiment 2
[0048] Embodiment 2: experiment in vivo
[0049] In the in vivo experiment, the MV4;11 CDX mouse model was constructed, and the number of cells injected into the tail vein of NSG mice d0 was 1×10 6 , The experiment was divided into 4 groups. Experimental group 1 was the Chelidonine treatment group, 7 animals in each group, and the survival time was observed and recorded; Experimental group 2 was the non-medication group, 7 animals in each group, and the survival time was observed and recorded; Experimental group 3 was Chelidonine drug treatment group. Treatment group, experimental group 4 is the non-medication group, and these two groups are the regular execution group. The incidence and tumor burden were measured after sacrifice to determine the therapeutic effect of Chelidonine in vivo. The earliest onset time of the established NSG mouse CDX model was d16 after transplantation, and the body weight decreased by 9% in 24 hours. Of the 6 NSG mice in the Chelidonine treatment...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com