Application and detection reagent for screening allopurinol tolerant gene methylation marker
An allopurinol and methylation technology, applied in the field of biotechnology, can solve problems such as severe drug eruption in patients, and achieve the effect of improving sensitivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Detection of HLA-B*5801 alleles in peripheral blood of allopurinol-induced severe drug eruption patients and allopurinol-resistant patients.
[0045] Genomic DNA was extracted from 2 mL of venous blood from each participant using the QIAamp DNA purification system (Qiagen). The presence or absence of HLA-B*58:01 was detected by the PG5801 DNA detection kit (Pharmigene), and the patients were further divided into four groups: those who carried the HLA-B*58:01 site (25 cases), those who did not Tolerant patients carrying the HLA-B*58:01 site (114 cases), severe drug eruption patients (116 cases) carrying the HLA-B*58:01 site, and those who did not carry the HLA-B*58:01 site Patients with severe drug eruption (8 cases). The kit relies on real-time polymerase chain reaction (PCR) with HLA-B*58:01 sequence-specific primers.
Embodiment 2
[0046] Example 2 Detection of genome-wide DNA methylation levels in peripheral blood of allopurinol-induced severe drug eruption patients and allopurinol-resistant patients.
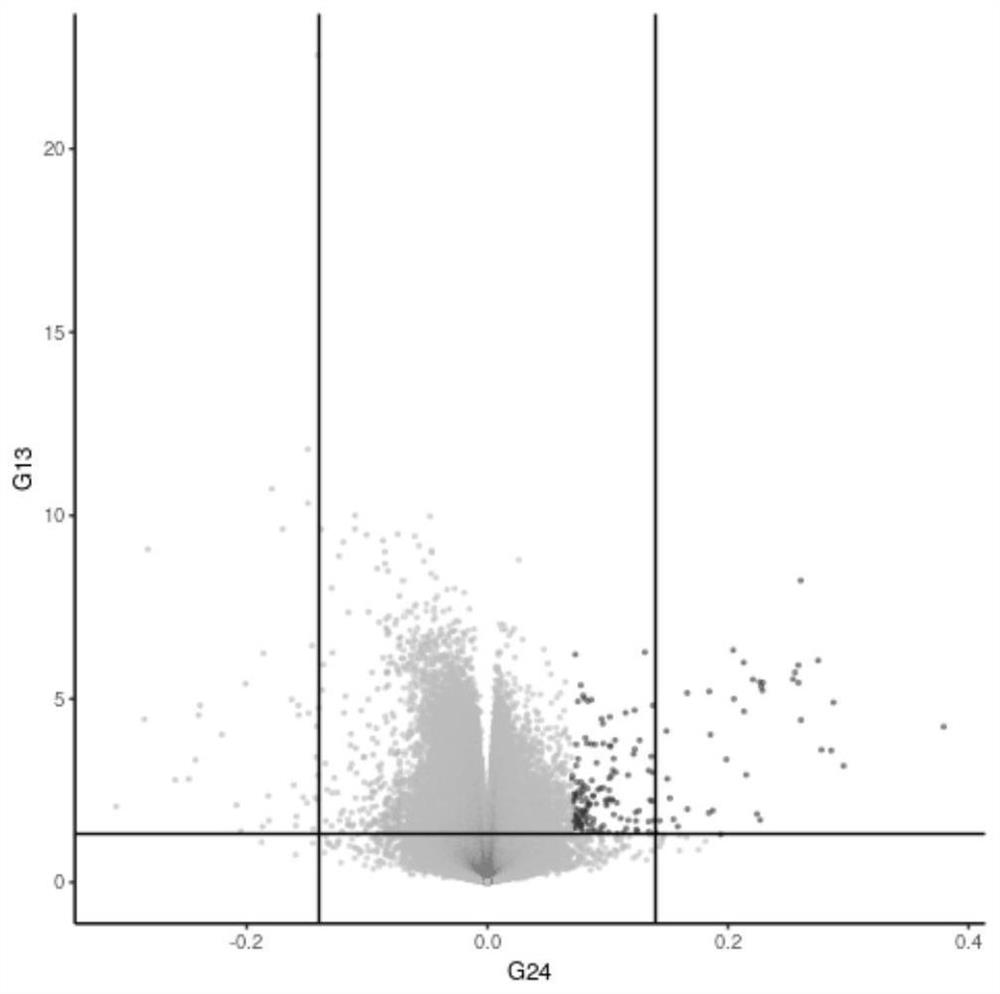
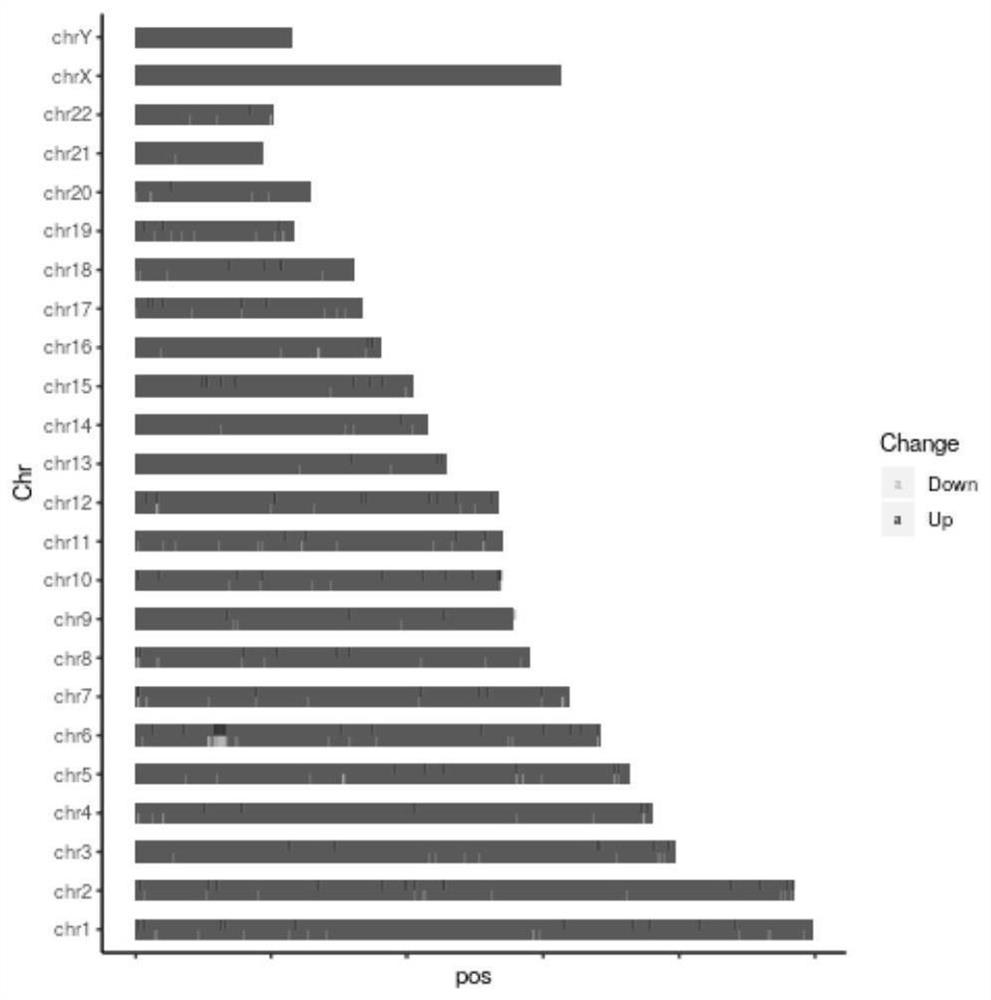
[0047] Four groups of patients with high DNA quality and matched gender and age were further screened for genome-wide DNA methylation level detection: tolerant patients carrying HLA-B*58:01 sites (18 cases), those without HLA-B *58:01 site tolerance (25 cases), severe drug eruption patients carrying HLA-B*58:01 site (13 cases), severe drug eruption patients not carrying HLA-B*58:01 site ( 6 cases). Extracted DNA samples were subjected to bisulfite conversion using the EZ DNA Methylation™ Kit (Zymo Research) according to the manufacturer's instructions. The transformed DNA was then applied to an Illumina Infinium Methylation Chip (850k). Differentially methylated CpGs were selected according to their beta values using the algorithm in IMA Bioconductor. Differential methylation was defined as an avera...
Embodiment 3
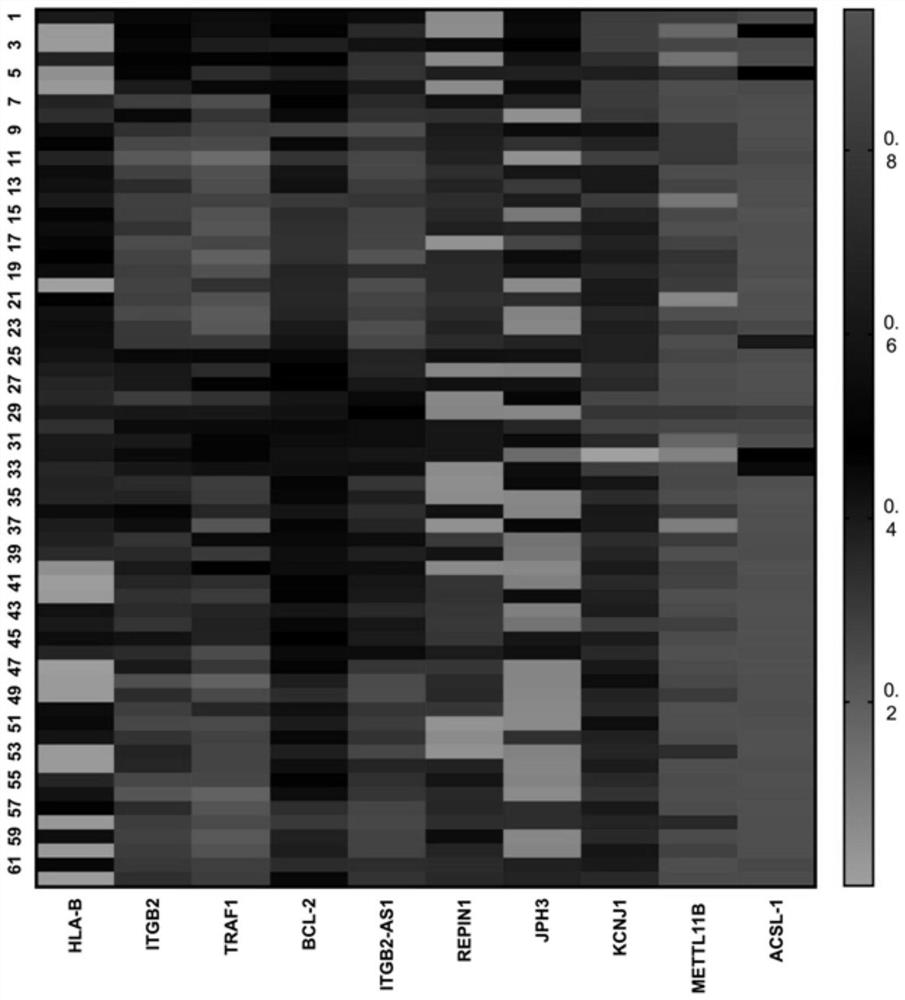
[0048] Example 3 analyzes the application of differentially methylated genes HLA-B, TRAF1 and ITGB2 in the screening of allopurinol-induced severe drug eruption patients and allopurinol-resistant patients.
[0049] The sensitivity and specificity of HLA-B, TRAF1 and ITGB2 methylation levels in screening allopurinol-induced severe drug eruption patients and allopurinol-resistant patients were calculated by ROC curve evaluation statistics. Analysis of tolerant individuals carrying HLA-B*58:01 (18 cases), tolerant individuals not carrying HLA-B*58:01 (25 cases), carrying HLA-B*58:01 The severe drug eruption patients (13 cases) and the severe drug eruption patients (6 cases) who did not carry the HLA-B*58:01 site. It is generally believed that the actual value range of the area under the ROC curve (AUC) is 0.5-1, and the diagnostic value is medium when it is 0.7-0.9, and the diagnostic value is high when it is above 0.9. HLA-B site methylation level distinguishes patients with se...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com