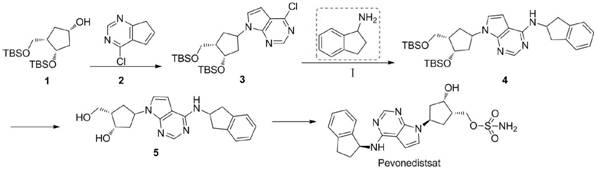
Electrochemical preparation method of rasagiline and Pevonedistat intermediates
An intermediate and electrode technology, applied in the field of electroreduction preparation, can solve the problems of large environmental pollution, excessive heavy metals, affecting the purity of intermediates, etc., and achieve the effects of simplifying the process flow, suitable for large-scale popularization and application, and reducing production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
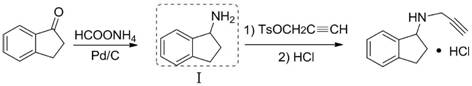
[0043] Preparation of 2,3-Dihydro-1H-inden-1-amine by Electroreduction
[0044]
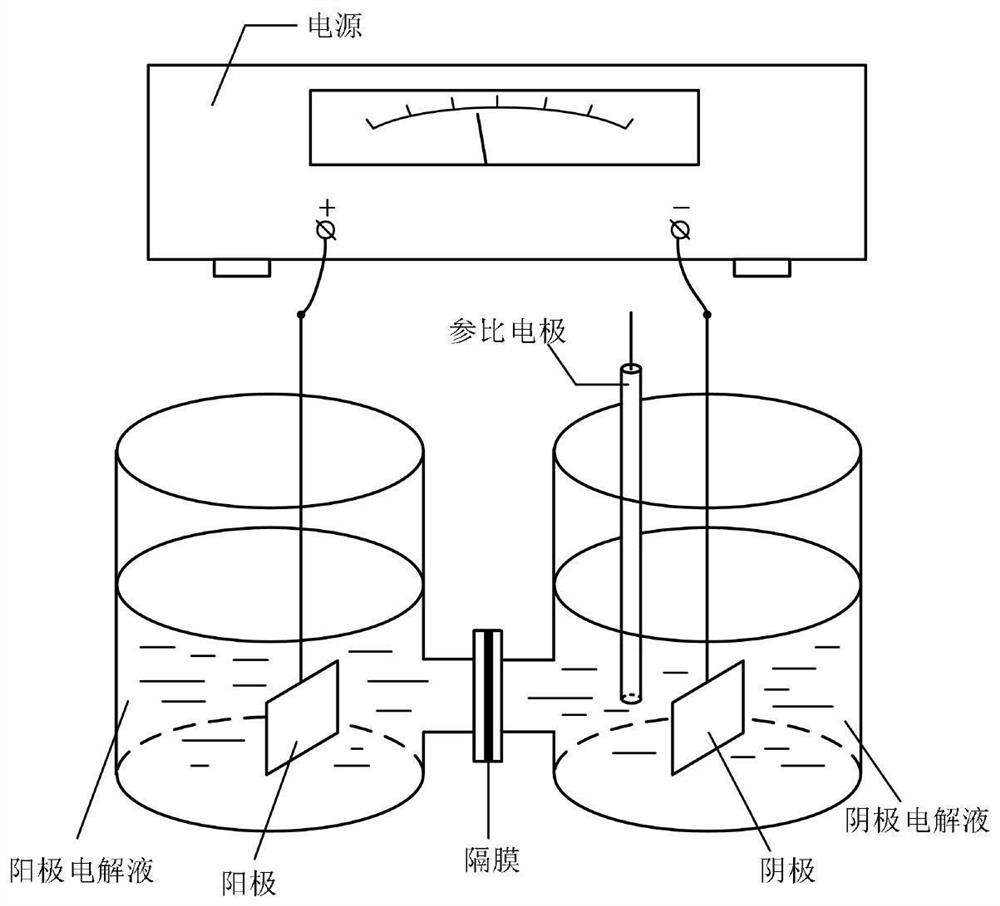
[0045] Divided electrolyzer ( figure 1 ), using a proton exchange membrane. At the cathode (zinc sheet 2×2cm 2) room, add 0.44g (3mmol) 2,3-dihydro-1H-inden-1-one oxime, 10mL methanol and 40mL0.2 mol / L KOH, anode room (platinum mesh 1×1cm 2 ) 50mL KOH solution with a concentration of 5mol / L; 50℃ constant current 0.6A reaction, current density 0.15A / cm 2 , the saturated calomel electrode is used as a reference electrode to monitor the cathode reaction potential, the cathode potential is 7.0-15.0V, the electroreduction reaction is 6.5h, the catholyte is extracted with ethyl acetate for 3 times, the organic layer is collected and dried over anhydrous sodium sulfate, and spin-evaporated to remove the solvent. An oily compound was obtained, which was formed into a salt by injecting HCl gas to obtain 0.46 g of 2,3-dihydro-1H-inden-1-amine hydrochloride (white solid), melting point 210-212°C, yiel...
Embodiment 2
[0047] Preparation of 2,3-Dihydro-1H-inden-1-amine by Electroreduction
[0048]
[0049] Divided electrolyzer ( figure 1 ), using a proton exchange membrane. At the cathode (zinc sheet 2×2cm 2 ) room, add 0.88g (6mmol) 2,3-dihydro-1H-inden-1-one oxime, 5mL methanol: 45mL0.5 mol / LKOH, anode room (platinum mesh 1×1cm 2 ) 50mL KOH solution with a concentration of 5mol / L; 55℃ constant current 0.6A reaction, current density 0.15A / cm 2 , the saturated calomel electrode is used as a reference electrode to monitor the cathode reaction potential, the cathode potential is 7.0-15.0V, the electroreduction reaction is 6.5h, the catholyte is extracted with ethyl acetate for 3 times, the organic layer is collected and dried over anhydrous sodium sulfate, and spin-evaporated to remove the solvent. The oily compound was obtained, and HCl gas was added to form a salt to obtain 0.92 g of 2,3-dihydro-1H-inden-1-amine hydrochloride (white solid), melting point 210-212°C, yield 90.6%.
Embodiment 3
[0050] Embodiment 3 (control experiment 1)
[0051] Reduction Preparation of 2,3-Dihydro-1H-inden-1-amine
[0052]
[0053] 2,3-Dihydro-1H-indene-1-amine was prepared according to the literature [Research on the synthesis process of the second generation monoamine oxidase inhibitor rasagiline mesylate [D]. Jilin University, 2018] method: 50.0g (0.34mol ) 2,3-dihydro-1H-inden-1-one oxime, 250mL of ethanol, add 150g of 20% NaOH aqueous solution, control the reaction temperature at 60-65°C, add 100g of nickel-aluminum alloy in batches, and control the addition time within 2h; After the addition, maintain the temperature at 60-65°C for 6 hours. Suction filtration after completion of the reaction, the obtained solution was extracted three times with dichloromethane, the organic phase was washed with water, the dichloromethane was concentrated to about half of the remaining volume, extracted twice with 4mol / L hydrochloric acid solution, the combined feed liquid was concentrated ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com