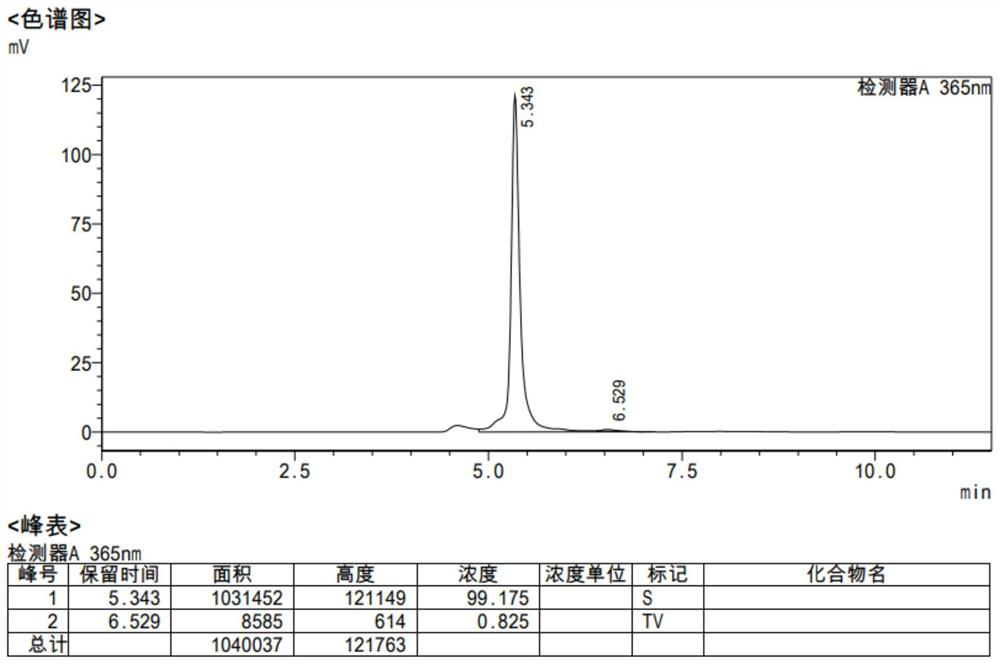
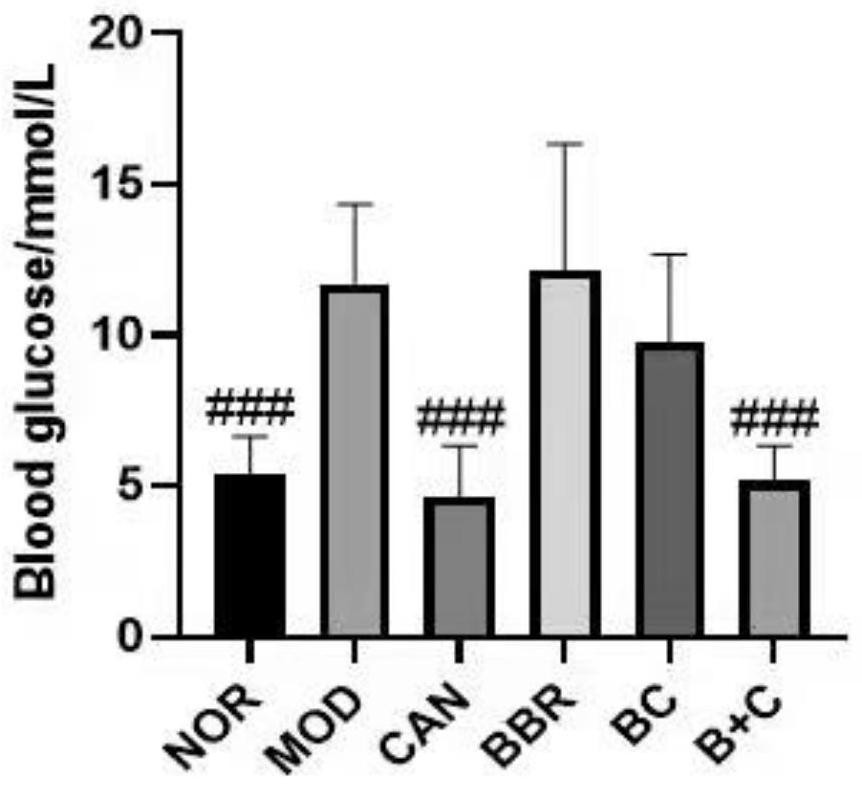
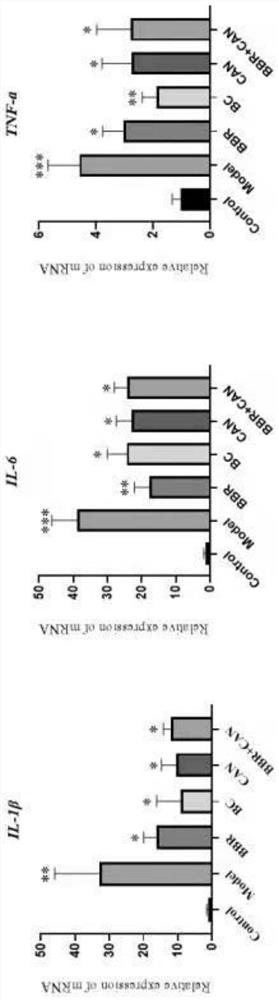
Berberine canagliflozin derivative as well as preparation method and application thereof
A technology of berberine and its derivatives, applied in the field of berberine canagliflozin derivatives and its preparation, achieving the effects of excellent antibacterial activity, less side reactions, and low requirements for reaction equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The following route (route 1) is designed:
[0043] Reaction 1: reduce berberine hydrochloride 1 to obtain reduced berberine (HB); after dissolving berberine hydrochloride (1g, 2.66mol) in 15ml pyridine, add 2eq of NaBH 4 , after reacting at room temperature for 1 h, product 2 reduced berberine can be obtained by washing with water, and the yield is 65%.
[0044]
[0045] In the preparation method of the above-mentioned reduced berberine: take berberine hydrochloride (1g, 2.66mol) and dissolve in 15ml pyridine (other acid-binding agents are also acceptable), add 1-4eq of NaBH 4 , after reacting at 0°C-25°C for 0.5-3h, the product reduced berberine 2 can be obtained by washing with water.
[0046] In this reaction method, berberine hydrochloride is dissolved in methanol solution containing potassium carbonate, and the reaction can also be carried out at 0° C., but it needs to be reacted for 8 hours, and the yield is 46%.
[0047] Reaction 2 (reaction step a): esteri...
Embodiment 2
[0056] In order to obtain the product 4 (BA-C), we continued to try to modify canagliflozin by protecting and deprotecting the hydroxysilyl ether, and made the following attempts (route 2):
[0057] Reaction 6 (reaction step e): the hydroxyl group at the canagliflozin hydroxysilyl ether is protected to obtain product 7;
[0058] Reaction 7 (reaction step f): product 7 is esterified to obtain product 8;
[0059] Reaction 8 (reaction step g): product 8 is deprotected to obtain product 9;
[0060] Reaction 9 (reaction step h): Bromination of product 9 affords product 10.
[0061]
[0062] It is worth mentioning that in Reaction 6, we tried to use TBDPSCl (tert-butyldiphenylchlorosilane), trimethylchlorosilane, TIPSCl (triisopropylchlorosilane) as protecting groups, and carried out TLC to the product system The spot plate found that the target substance could be detected only when TBDPSCl was used as the protecting group. Reaction 7 successfully detected the target product w...
Embodiment 3
[0064] After many attempts, finally use the synthesis of canagliflozin that is directly brominated and then esterified (route 3):
[0065] Reaction 10 (reaction step i): Synthesis of canagliflozin derivatives, reagents and conditions: i) CBr4, PPh3, Py, Ar, 50°C
[0066]
[0067] Reaction 10: Dissolve canagliflozin (1 mmol) in 2 ml of ultra-dry pyridine, start stirring under the protection of argon, and add triphenylphosphine (2 mmol). The temperature of the reaction solution was lowered to 0°C, and carbon tetrabromide (1 mmol) was slowly added to the reaction system. After the dropwise addition, the reaction was carried out at 50°C for 4 hours, and the product was separated by column chromatography. The developer was dichloromethane: Methanol 40:1, but preliminary characterization by NMR, not the target product.
[0068] Reaction 11 (reaction step j and reaction step k): synthesis of canagliflozin derivatives, reagents and conditions: j) NBS, PPh 3 , DMF, Ar, 50℃, 4h; k)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com