Hydrofining method of crude 2, 6-naphthalic acid
A naphthalene dicarboxylic acid, hydrogenation refining technology, applied in the direction of carboxylate preparation, chemical instruments and methods, preparation of organic compounds, etc., can solve the problem of high naphthalene production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
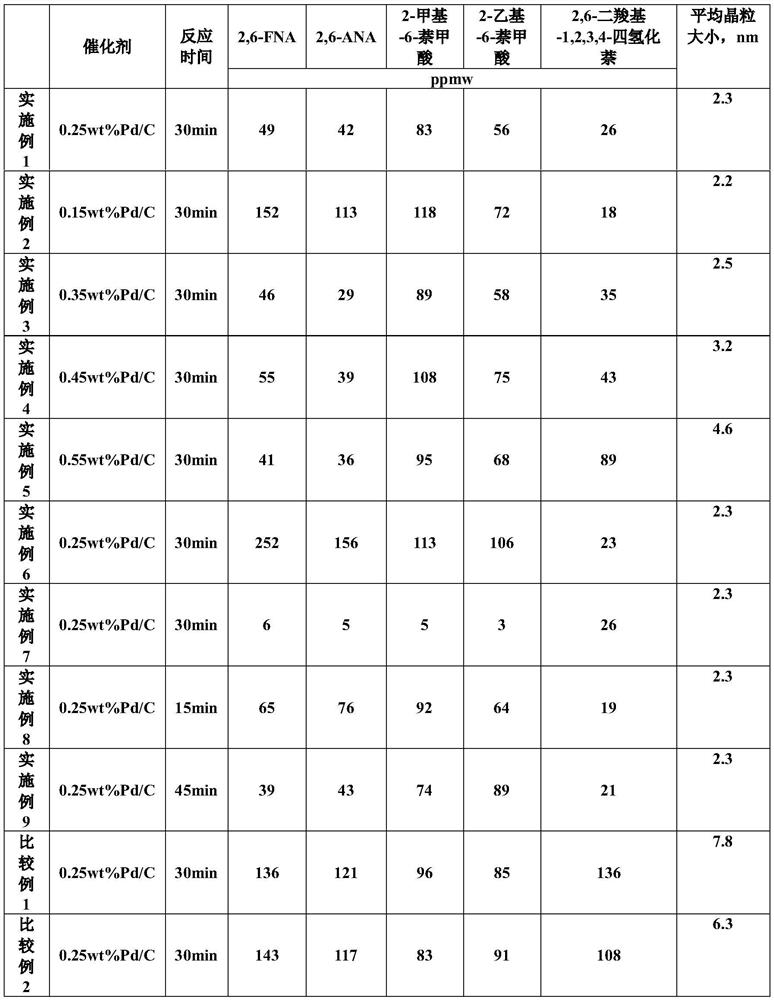
[0033] 1. Preparation of crude 2,6-naphthalenedicarboxylic acid samples
[0034] 311.25g Co(OAc) 2 ·4H 2 O, 306.25g Mn(OAc) 2 ·4H 2 O, 297.5g KBr, 367.5g CH 3 COOK and 7760g acetic acid are mixed and added in the reactor, then open and stir and be warming up to 185 ℃, the reactor pressure is controlled at 2.75MPa, 1000g 2,6-diisopropylnaphthalene is first warming up to molten state, then with 6g / min The speed enters the reaction kettle, and at the same time, a sufficient amount of air is introduced for the reaction. After the feeding is completed, the reaction temperature and pressure are maintained to continue the reaction for 1 h. Filter, and respectively adopt 60 ℃ of acetic acid washing, 80 ℃ of water washing, the consumption of washing acetic acid is 1000g, and the consumption of washing water is 1000g, after sampling and drying, analyze the impurities 2-formyl-6-naphthoic acid and 2-acetyl- 6-naphthoic acid content. After analysis, the content of 2-formyl-6-naphtho...
Embodiment 2
[0041] 1. Preparation of crude 2,6-naphthalenedicarboxylic acid samples
[0042] 311.25g Co(OAc) 2 ·4H 2 O, 306.25g Mn(OAc) 2 ·4H 2 O, 297.5g KBr, 367.5g CH 3 COOK and 7760g acetic acid are mixed and added in the reactor, then open and stir and be warming up to 185 ℃, the reactor pressure is controlled at 2.75MPa, 1000g 2,6-diisopropylnaphthalene is first warming up to molten state, then with 6g / min The rate enters the reaction kettle, and at the same time, a sufficient amount of air is introduced for the reaction. After the feeding is completed, the reaction temperature and pressure are maintained to continue the reaction for 1 h. Filter, and adopt 60 ℃ of acetic acid washing respectively, 80 ℃ of water washing, the consumption of washing acetic acid is 1000g, the consumption of washing water is 1000g, after sampling and drying, analyze the impurities 2-formyl-6-naphthoic acid and 2-acetyl- 6-naphthoic acid content. After analysis, the content of 2-formyl-6-naphthoic ac...
Embodiment 3
[0049] 1. Preparation of crude 2,6-naphthalenedicarboxylic acid samples
[0050] 311.25g Co(OAc) 2 ·4H 2 O, 306.25g Mn(OAc) 2 ·4H 2 O, 297.5g KBr, 367.5g CH 3 COOK and 7760g acetic acid are mixed and added in the reactor, then open and stir and be warming up to 185 ℃, the reactor pressure is controlled at 2.75MPa, 1000g 2,6-diisopropylnaphthalene is first warming up to molten state, then with 6g / min The rate enters the reaction kettle, and at the same time, a sufficient amount of air is introduced for the reaction. After the feeding is completed, the reaction temperature and pressure are maintained to continue the reaction for 1 h. Filter, and adopt 60 ℃ of acetic acid washing respectively, 80 ℃ of water washing, the consumption of washing acetic acid is 1000g, the consumption of washing water is 1000g, after sampling and drying, analyze the impurities 2-formyl-6-naphthoic acid and 2-acetyl- 6-naphthoic acid content. After analysis, the content of 2-formyl-6-naphthoic ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com