Catalyst and method for preparing hydroxyaldehyde by olefine aldehyde hydration
A technology for the hydration of alkenes and hydroxyaldehydes, which is applied to the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds. It can solve problems such as high reaction temperature, reduced conversion rate and selectivity, and unfavorable industrialization. Catalyst pulverization, low reaction temperature, and reduced bed pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~8
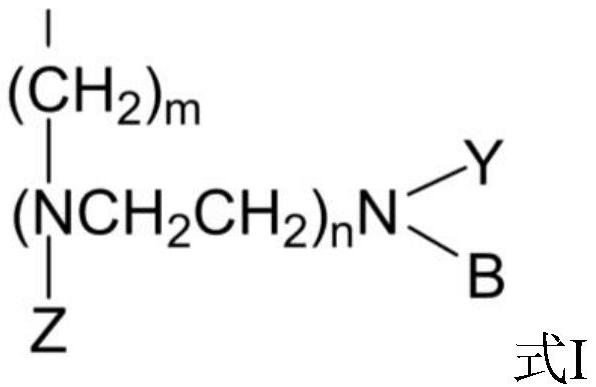
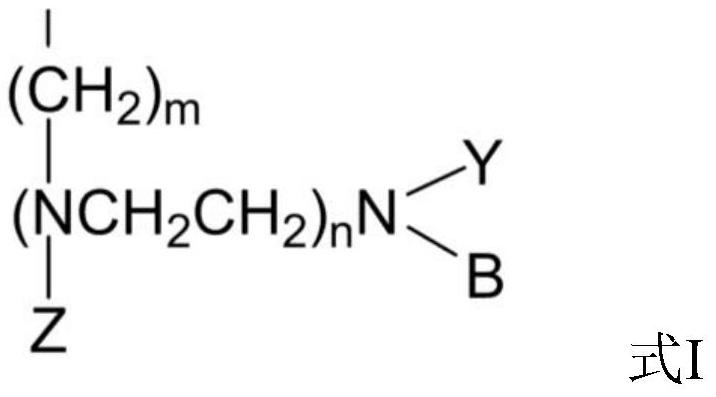
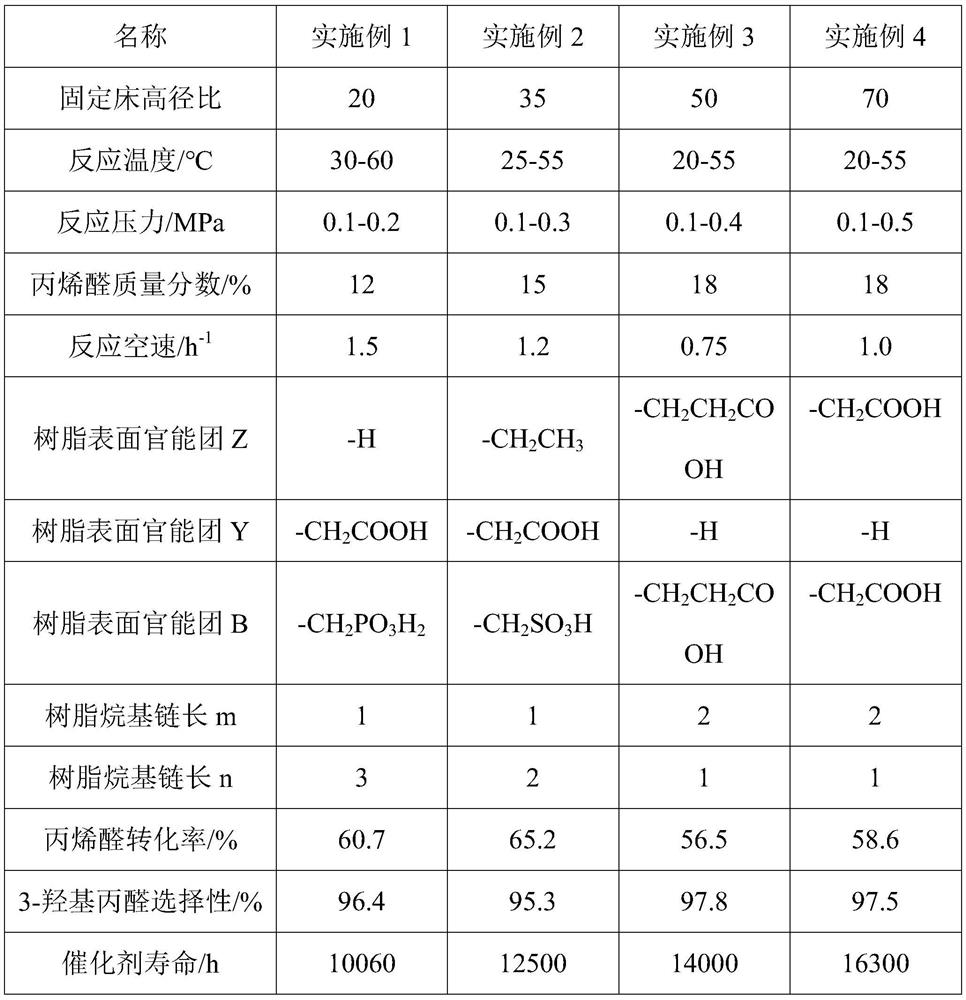
[0061] Embodiments 1 to 8 respectively provide a method for preparing 3-hydroxypropanal by hydration of acrolein, the method comprising:
[0062] Load a certain quality resin catalyst containing polyamine acidic functional groups into (length) *14mm (inner diameter) fixed-bed reactor, through the catalyst loading, control different aspect ratios, use a metering pump to feed 10-20% acrolein aqueous solution for 0.5-2.0h -1 Pump into the catalyst bed for acrolein hydration reaction, the temperature of the initial preheater and fixed bed insulation jacket is 20°C, the reaction pressure is normal pressure, and the conversion rate of acrolein is controlled to be ≥ 50%, such as the concentration of acrolein < 50% , then increase the temperature of the preheater and the jacket, each time by 1 °C, so that the conversion rate of acrolein is increased to more than 50%. The temperature is 60°C. The hydration liquid obtained from the hydration reaction was cooled by a condenser at 5°C ...
Embodiment 9
[0068] This example provides a method for preparing 3-hydroxypropanal by hydration of acrolein. The method is the same as that of Example 1 except that the reaction is carried out at 20°C without raising the temperature.
Embodiment 10
[0070] This example provides a method for preparing 3-hydroxypropanal by hydration of acrolein. Except that the method is directly preheated to 52°C and reacted at 52°C, and the temperature is not raised during the hydration reaction, the rest are the same as in Example 1. same.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com