Tosufloxacin tosylate and preparation method thereof
A technology of tosufloxacin tosylate and tosufloxacin tosylate, which is applied in the field of medicine, can solve problems such as low yield, low purity, and no ideal synthesis route of tosufloxacin tosylate, and achieve high yield The effect of high and low impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] This embodiment provides a preparation method of tosufloxacin tosylate. The preparation method of tosufloxacin tosylate of the present embodiment may further comprise the steps:
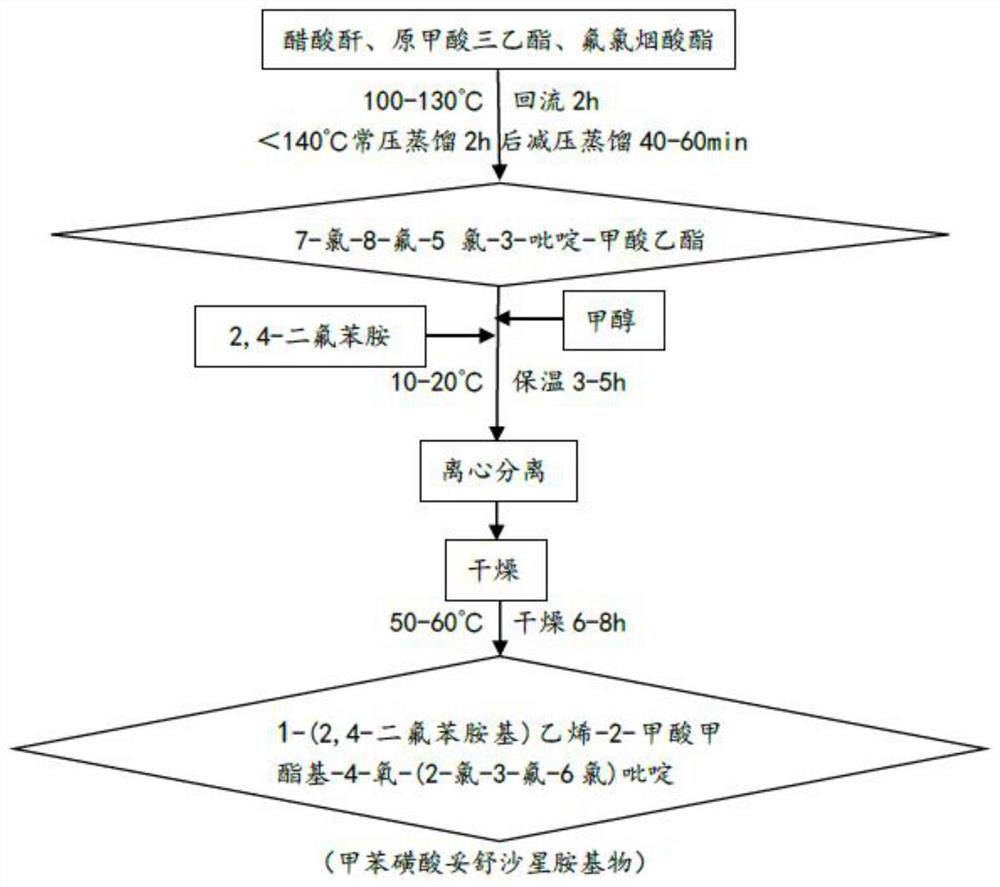
[0034] Amination: Tosufloxacin tosylate amine was prepared by amination reaction using acetic anhydride, triethyl orthoformate, fluclonicotinate, methanol, and 2,4-difluoroaniline as starting materials;
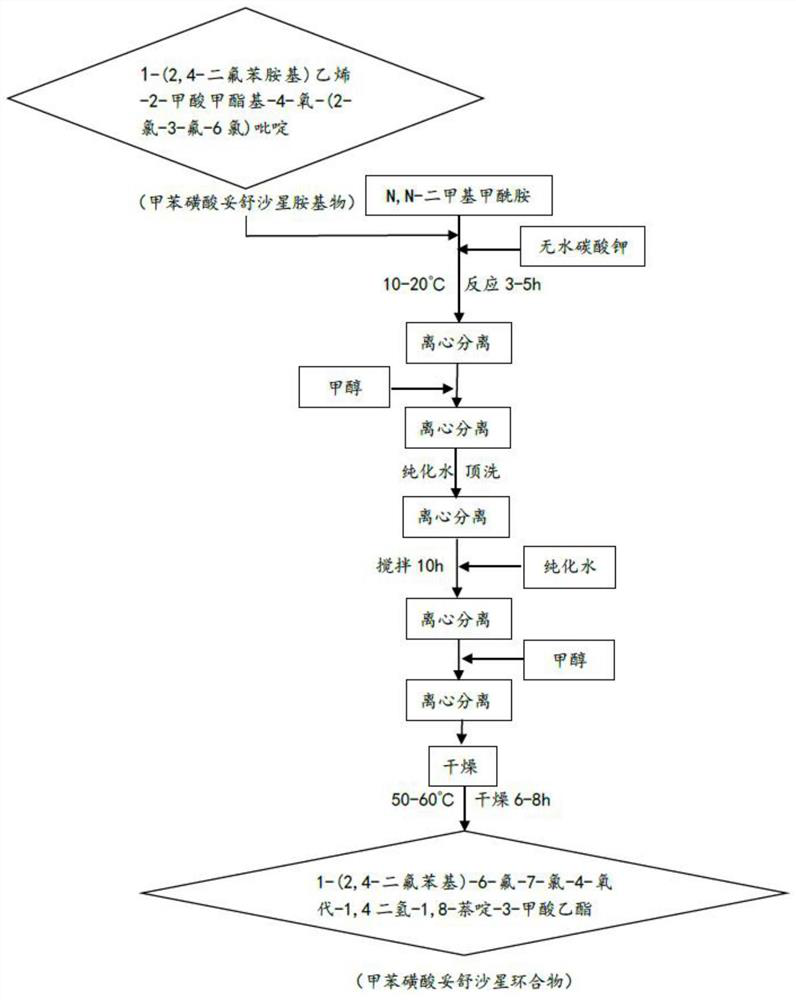
[0035] Cyclization: based on tosufloxacin tosylate amine, prepare tosufloxacin tosylate cyclic compound through cyclization reaction;
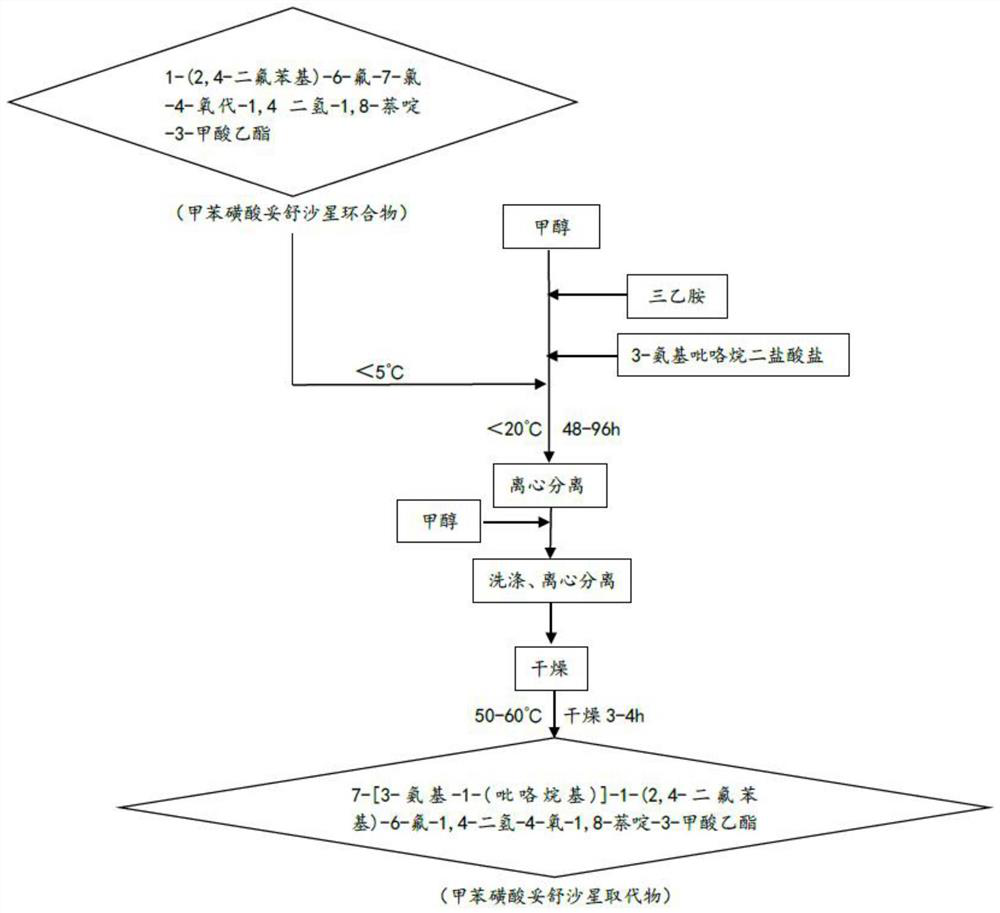
[0036] Substitution: Based on the tosufloxacin tosylate cyclic compound, the tosufloxacin tosylate substituted was prepared through a substitution reaction;
[0037] Hydrolysis into salt: Based on the tosufloxacin toluenesulfonate substitute, the crude product of tosufloxacin toluenesulfonate is prepared by hydrolysis into salt, and the refined tosufloxacin toluenesulfonate is obtained.
[0038] In this example, tosufloxacin tosylate amine is 1-(2,4-difluor...
Embodiment 2
[0093] This embodiment provides a kind of tosufloxacin tosylate and its preparation method. The same technical features of the preparation method of this embodiment and embodiment 1 will not be repeated, and only the difference with embodiment 1 will be described:
[0094] (1) Tosufloxacin tosylate amination post:
[0095] Reaction process: in the present embodiment, the charging capacity of fluchloronic acid ester is 300.0kg (1.0714kmol), the charging capacity of acetic anhydride is 270.0kg (2.6447kmol), the charging capacity of triethyl orthoformate is 225.0kg ( 2.4433kmol), the feeding amount of 2,4-difluoroaniline is 153.75kg (1.4914kmol), and the feeding amount of methanol is 900.0kg (28.0899kmol).
[0096] The reaction process comprises the following steps: use a vacuum pump to sequentially draw acetic anhydride and triethyl orthoformate into the reaction tank from the liquid feed port, start stirring, add fluchloronicotinic acid ester from the solid feed port, after th...
Embodiment 3
[0115] This embodiment provides a kind of tosufloxacin tosylate and its preparation method. The same technical features of the preparation method of this embodiment and embodiment 1 will not be repeated, and only the difference with embodiment 1 will be described:
[0116] (1) Tosufloxacin tosylate amination post:
[0117] Reaction process: In the present embodiment, the feeding amount of fluchloronic acid ester is 200kg, the feeding amount of acetic anhydride is 180kg, the feeding amount of triethyl orthoformate is 150kg, and the feeding amount of 2,4-difluoroaniline is 102.5kg, the feeding amount of methanol is 600kg.
[0118] The reaction process comprises the following steps: use a vacuum pump to sequentially draw acetic anhydride and triethyl orthoformate into the reaction tank from the liquid feed port, start stirring, add fluchloronicotinic acid ester from the solid feed port, after the feeding is completed, seal the tank, and the temperature rises to 120°C, reflux re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com