Method for promoting bisphenol A conversion by laccase-induced copolymerization
A technology of copolymerization and laccase, which is applied in the field of cross fusion, can solve the problems of decreased catalytic activity of laccase, hindering the enzymatic reaction rate and removal effect of BPA monomer, and inactivation, so as to reduce the mineralization of microorganisms and eliminate biological Toxic effects, effects of rapid oxidation and removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1H
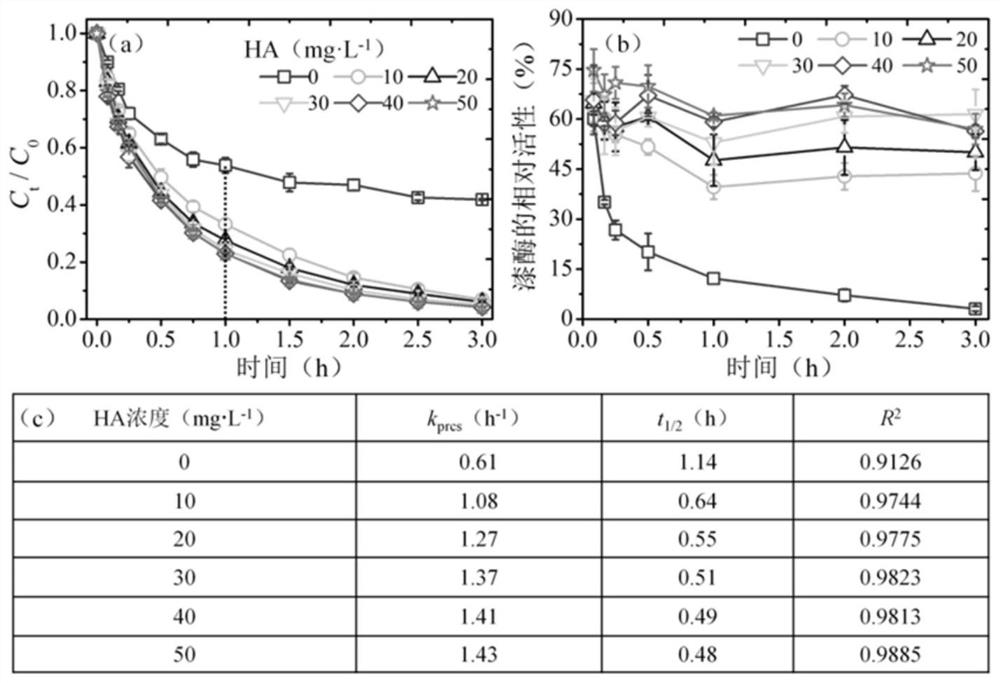
[0021] Example 1 Effect of HA on the oxidation and removal of BPA induced by versicolor laccase
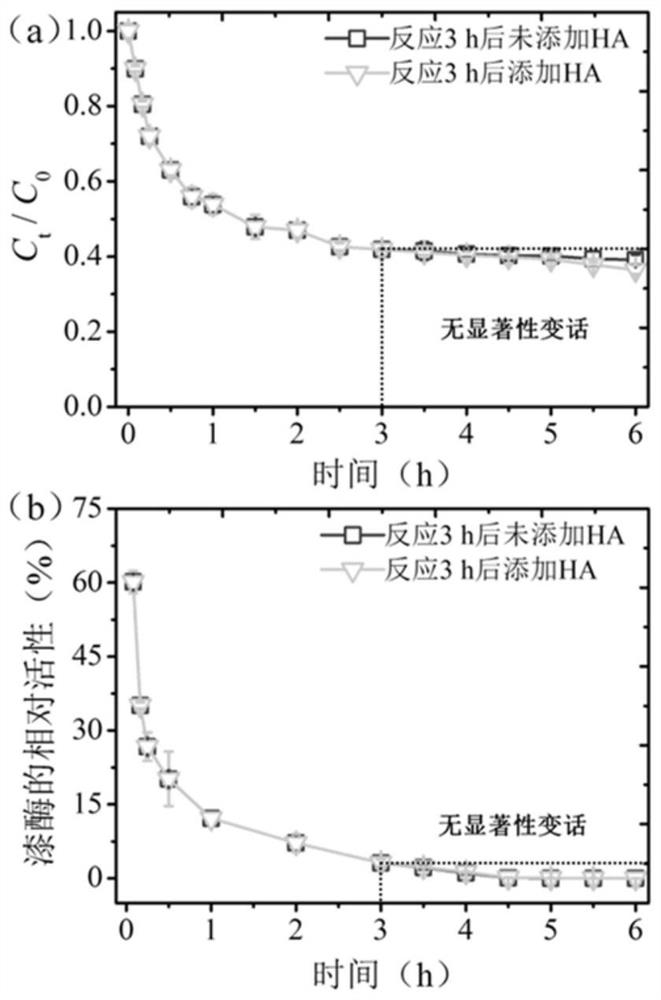
[0022] To investigate the effect of HA concentration on the conversion of BPA induced by T.versicolor laccase. The key experimental steps are briefly described as follows: (1) A 50mL heat-resistant brown glass bottle was selected as a container for laccase to initiate free radical polymerization, and the container contained 20mL of 10mM citric acid-phosphate buffer, 100μM BPA and 0-50 (ie 0, 10, 20, 30, 40 and 50) mg·L -1 HA; (2) Accurately add 2U·mL to the buffer -1 Yunzhi laccase (light brown, ≥0.5U·mg -1 ), start the enzymatic polymerization reaction; (3) the condition of free radical polymerization initiated by Yunzhi laccase is pH 5.0, 25°C, and dark conditions, and stand for oxidative polymerization for 3 hours; (4) In addition, after 3 hours of enzymatic reaction, Add 30mg·L to the system -1 HA, to further study the effect of HA addition sequence on laccase-induced BPA ...
Embodiment 2
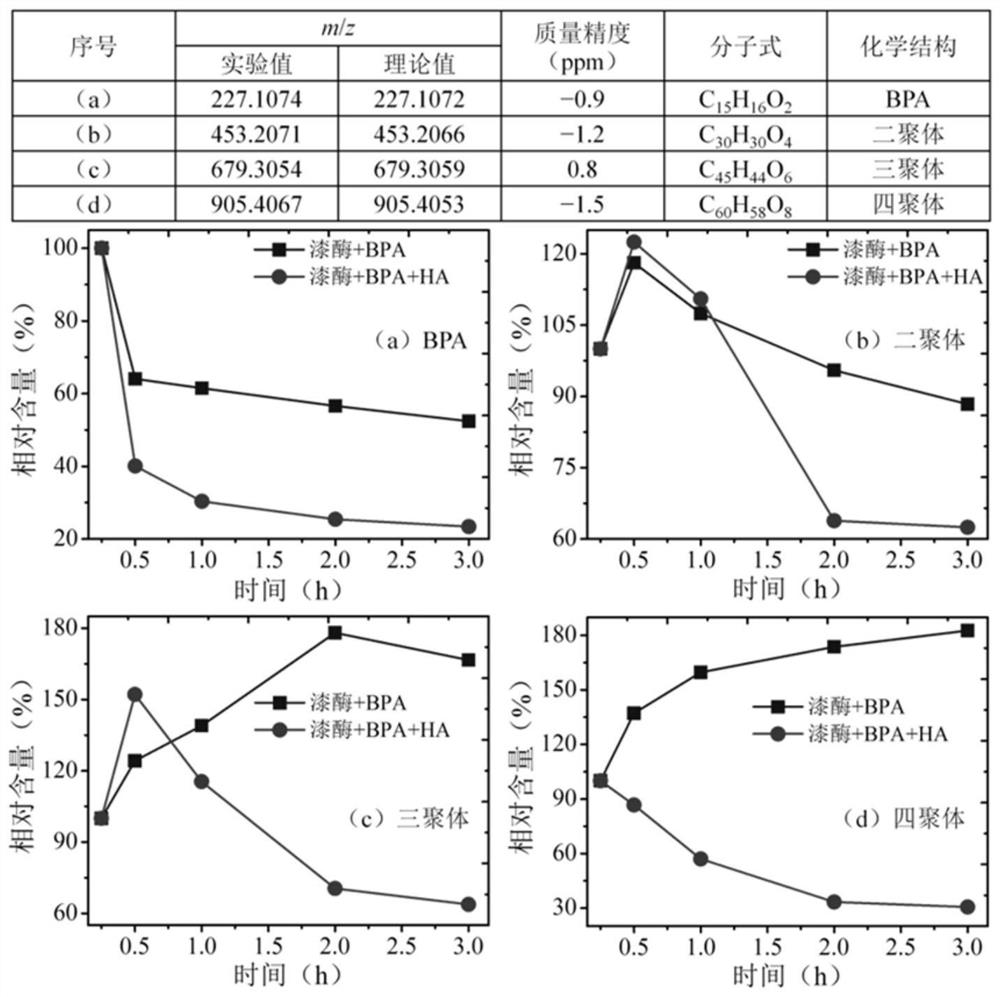
[0024] The influence of embodiment 2HA on BPA autopolymer output and distribution
[0025] Using LTQ-Orbitrap high-resolution mass spectrometry (HRMS, Thermo Fisher Scientific, Bremen, Germany; molecular mass accuracy -1) on the production and distribution of BPA autopolymer induced by Yunzhi laccase. A total of three macromolecular BPA self-polymers were screened out, and their m / z were 453.2071, 679.3054 and 905.4067, respectively. Based on the precise molecular masses and elemental compositions of the three compounds, these products were preliminarily identified as BPA dimers (C 30 h 30 o 4 ), trimer (C 45 h 44 o 6 ) and tetramers (C 60 h 58 o 8 ) (molecular mass accuracy <2.0ppm). It should be pointed out that the generation of small molecular BPA degradation products and supramolecular BPA-HA copolymers were not detected. Mainly because of the complexity and uncertainty of the structure of HA, it is difficult to reveal the molecular mass and chemical structure o...
Embodiment 3
[0027] The physical and chemical properties of embodiment 3 supramolecular BPA-HA copolymer
[0028] A large number of supramolecular BPA-HA copolymer particle products visible to the naked eye were obtained by using a continuous large-scale test method, and the basic physical and chemical properties of the supramolecular BPA-HA copolymer were analyzed. The main experimental operation steps are briefly described as follows: (1) The reactor contains 1L of 10mM citric acid-phosphate buffer, 100μM BPA, 30mg·L -1 HA and 2U·mL -1 Yunzhi laccase; (2) Place the reaction system in a dark place at 25°C and pH 5.0, 150r·min -1 After enzymatic oxidative polymerization for 3 hours, immediately add high-concentration hydrochloric acid to acidify the enzymatic reaction solution to pH 1.0; this process can not only quench the active phenol radical intermediate, but also promote the precipitation and precipitation of supramolecular BPA-HA copolymer; (3) The reaction solution quenched by the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com