Telmisartan oral solid preparation with stable product performance and preparation method of telmisartan oral solid preparation
A technology for telmisartan and solid preparations, applied in the field of telmisartan oral solid preparations and its preparation, can solve the problems of low tablet dissolution efficiency, unstable dissolution performance, and low bioavailability, and achieve optimized content uniformity , provide compressibility, and optimize the effect of inlet air temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
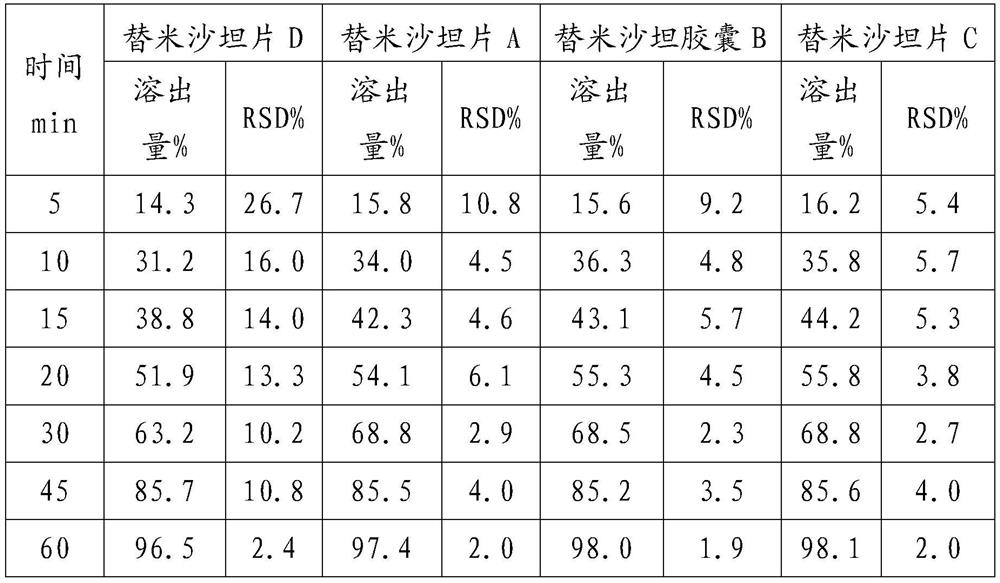
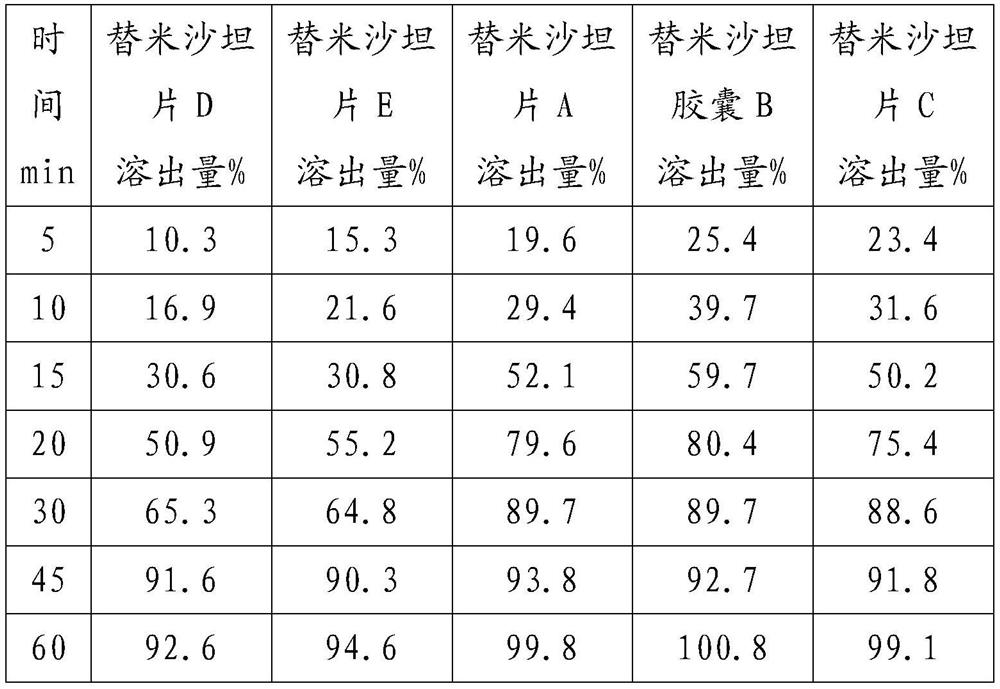
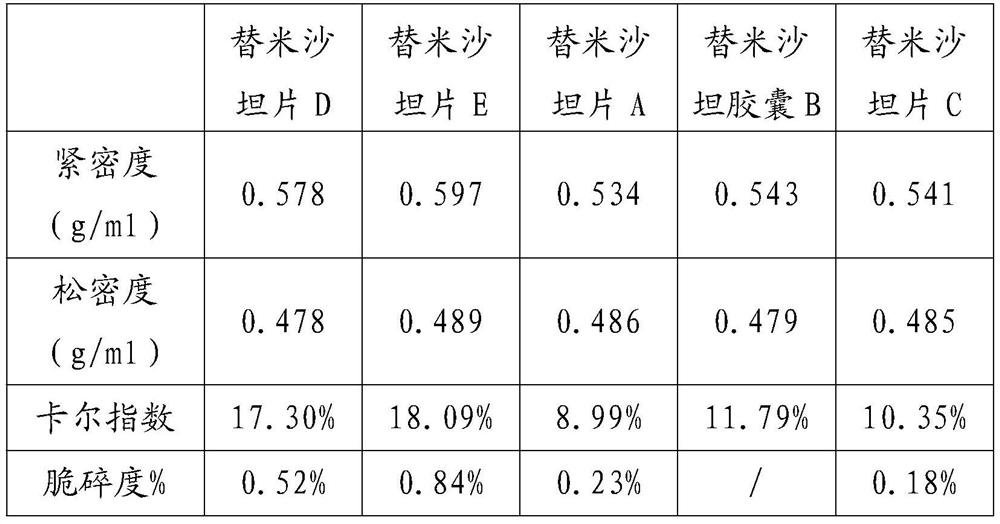
[0028] The formula (based on 1000 tablets, unit g) is as follows:
[0029] Telmisartan 80 sodium hydroxide 6.72 Povidone K30 24 meglumine 24 Hypromellose 24 Mannitol core 292.08 Magnesium stearate 4.8 talcum powder 9.6 silica 4.8
[0030] Add sodium hydroxide and meglumine to a certain amount of purified water to dissolve, then add the telmisartan raw material into the solution and stir, after it is completely dissolved, continue to add povidone, stir to dissolve, and clear and transparent A slurry is prepared. Add the formula amount of mannitol ball core into the fluidized bed, set the air intake to 70°C, the air volume to 2500m3 / h, the spraying speed to 150r / min, and the material temperature to be controlled at about 42°C. The prepared granules are sized, mixed with additional auxiliary materials, and then compressed into tablets to obtain Telmisartan tablets A.
Embodiment 2
[0032] Granules were prepared according to Example 1, except that capsules were used for filling to obtain Telmisartan capsule B.
Embodiment 3
[0034] Prepare particles according to Example 1, the difference is that the air intake volume is set to 2800m 3 / h, spray speed 170r / min, made telmisartan sheet C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| friability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com